Abstract
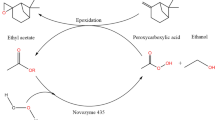
The use of immobilized lipase from Candida antarctica (Novozym® 435) to catalyze acetylation of trans-3,5,4′-trihydroxystilbene was investigated in this study. Response surface methodology and 5-level-4-factor central composite rotatable design were adopted to evaluate the effects of synthesis variables, including reaction time (24–72 h), temperature (25–65 °C), substrate molar ratio (1:15–1:75), and enzyme amount (600–3,000 PLU) on the percentage molar conversion of trans-4′-O-acetyl-3,5-dihydroxystilbene. The results showed that reaction temperature and enzyme amount were the most important parameters on percentage molar conversion. Based on ridge max analysis, the optimum conditions for synthesis were: reaction time 60 h, reaction temperature 64 °C, substrate molar ratio 1:56 and enzyme amount 2,293 PLU. The molar conversion of actual experimental values was 95% under optimal conditions. The synthesis product was analyzed using HPLC, mass and NMR. The results revealed that the major product was trans-4′-O-acetyl-3,5-dihydroxystilbene. The reaction kinetics was found to follow the **-Pong mechanism; substrate inhibition was not found at high vinyl acetate concentration.






Similar content being viewed by others
References
Stasiuk M, Kozubek A (2010) Biological activity of phenolic lipids. Cell Mol Life Sci 67:841–860
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821–1835
Tosun I, Inkaya AN (2010) Resveratrol as a health and disease benefit agent. Food Rev Int 26:85–101
Shukla Y, Singh R (2011) Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci 1215:1–8
Cardile V, Lombardo L, Spatafora C, Tringali C (2005) Chemo-enzymatic synthesis and cell-growth inhibition activity of resveratrol analogues. Bioorganic Chem 33:22–33
Gelo Pujic M, Desmurs JR, Kassem T, Delaire S, Adao A, Tawil D (2008) Synthesis of new antioxidant conjugates and their in vitro hydrolysis with stratum corneum enzymes. Int J Cosmet Sci 30:195–204
Nicolosi G, Spatafora C, Tringali C (2002) Chemo-enzymatic preparation of resveratrol derivatives. J Mol Catal B Enzym 16:223–229
Torres P, Poveda A, Jimenez-Barbero J, Ballesteros A, Plou FJ (2010) Regioselective lipase-catalyzed synthesis of 3-o-acyl derivatives of resveratrol and study of their antioxidant properties. J Agric Food Chem 58:807–813
Teng RW, Bui TKA, McManus D, Armstrong D, Mau SL, Bacic A (2005) Regioselective acylation of several polyhydroxylated natural compounds by Candida antarctica lipase B. Biocatal Biotransform 23:109–116
Trötzmüller M, Guo X, Fauland A, Köfeler H, Lankmayr E (2011) Characteristics and origins of common chemical noise ions in negative ESI LC–MS. J Mass Spectrom 46:553–560
SAS (1990) SAS User’s Guide. SAS Institute, Cary
Wong Y, Osmond G, Brewer KI, Tyler DS, Andrus MB (2010) Synthesis of 4′-ester analogs of resveratrol and their evaluation in malignant melanoma and pancreatic cell lines. Bioorg Med Chem Let 20:1198–1201
Yang H, Baur JA, Chen A, Miller C, Sinclair DA (2007) Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell 6:35–43
Guyot B, Bosquette B, Pina M, Graille J (1997) Esterification of phenolic acids from green coffee with an immobilized lipase from Candida antarctica in solvent-free medium. Biotechnol Lett 19:529–532
Karboune S, Safari M, Lue BM, Yeboah FK, Kermasha S (2005) Lipase-catalyzed biosynthesis of cinnamoylated lipids in a selected organic solvent medium. J Biotechnol 119:281–290
Liu KJ, Huang YR (2010) Lipase-catalyzed production of a bioactive terpene ester in supercritical carbon dioxide. J Biotechnol 146:215–220
Song QX, Wei DZ (2002) Study of Vitamin C ester synthesis by immobilized lipase from Candida sp. J Mol Catal B Enzym 18:261–266
Yadav GD, Lathi PS (2005) Lipase catalyzed transesterification of methyl acetoacetate with n-butanol. J Mol Catal B Enzym 32:107–113
Alberty RA (1953) The relationship between Michaelis constants, maximum velocities and the equilibrium constant for an enzyme-catalyzed reaction. J Am Chem Soc 75:1928–1932
Kuo CH, Chen CC, Chiang BH (2004) Process characteristics of hydrolysis of chitosan in a continuous enzymatic membrane reactor. J Food Sci 69:E332–E337
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kuo, CH., Hsiao, FW., Dai, SM. et al. Lipase catalyzed acetylation of 3,5,4′-trihydroxystilbene: optimization and kinetics study. Bioprocess Biosyst Eng 35, 1137–1145 (2012). https://doi.org/10.1007/s00449-012-0698-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0698-0