Abstract
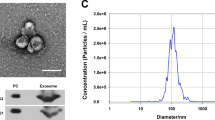
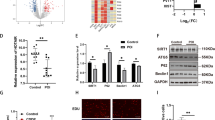
The role of plasma-derived exosomal miRNA in premature ovarian failure (POF) remains unclear. This study aimed to investigate the epigenetic pathogenesis of POF through exosomal miRNA sequencing. Exosomes were isolated and characterized from six POF patients and four healthy individuals using nanoparticle tracking analysis, transmission electron microscopy and western blot analysis. Exosomal miRNA sequencing was performed to identify differentially expressed miRNAs with |fold change| greater than 1.5 and p value less than 0.05. Bioinformatics analysis in GSE39501 dataset and our sequencing data was conducted to investigate underlying mechanisms of POF. The functional role of hsa-miR-19b-3p was assessed using CCK8, western blot, flow cytometry and fluorescence staining. The regulatory effect of hsa-miR-19b-3p on BMPR2 was investigated through miRNA transfection, qPCR analysis, and luciferase reporter assay. Statistical significance was determined using t-tests and one-way ANOVA (p < 0.05). Exosomal miRNA sequencing revealed 18 dysregulated miRNAs in POF patients compared to healthy controls. Functional enrichment analysis demonstrated their involvement in cell growth, oocyte meiosis and PI3K-Akt signaling pathways. Moreover, the constructed miRNA–mRNA network unveiled potential regulatory mechanisms underlying POF, particularly implicating hsa-miR-19b-3p in the regulation of BMPR2. In vitro assays conducted on KGN cells confirmed that hsa-miR-19b-3p promoted apoptosis, as evidenced by reduced cell viability, decayed mitochondrial membrane potential and increased apoptotic rate, thereby supporting its role in POF. Notably, hsa-miR-19b-3p was found to significantly downregulate BMPR2 expression via targeting its 3′UTR, while co-expression analysis revealed strong associations between BMPR2 and POF-related processes. This study sheds light on the epigenetic pathogenesis of POF by investigating exosomal miRNA profiles. Particularly, hsa-miR-19b-3p emerged as a potential regulator of BMPR2 and demonstrated its functional significance in POF through modulation of apoptosis.






Similar content being viewed by others
Availability of data and materials
The sequencing data utilized in this study are accessible through online repositories named NCBI GEO, with the accession number GSE229434. The token number for accessing data is: irgpquamjvulhev.
References
Cai JH, Sun YT, Bao S (2022) HucMSCs-exosomes containing miR-21 promoted estrogen production in ovarian granulosa cells via LATS1-mediated phosphorylation of LOXL2 and YAP. Gen Comp Endocrinol 321–322:114015. https://doi.org/10.1016/j.ygcen.2022.114015
Cui L, Bao H, Liu Z, Man X, Liu H, Hou Y, Luo Q, Wang S, Fu Q, Zhang H (2020) hUMSCs regulate the differentiation of ovarian stromal cells via TGF-β (1)/Smad3 signaling pathway to inhibit ovarian fibrosis to repair ovarian function in POI rats. Stem Cell Res Ther 11:386. https://doi.org/10.1186/s13287-020-01904-3
Dastmalchi N, Safaralizadeh R, Khojasteh SMB, Shadbad MA, Hosseinpourfeizi MA, Azarbarzin S, Rajabi A, Baradaran B (2022) The combined restoration of miR-424-5p and miR-142-3p effectively inhibits MCF-7 breast cancer cell line via modulating apoptosis, proliferation, colony formation, cell cycle and autophagy. Mol Biol Rep 49:8325–8335. https://doi.org/10.1007/s11033-022-07646-0
Esfandyari S, Elkafas H, Chugh RM, Park HS, Navarro A, Al-Hendy A (2021) Exosomes as biomarkers for female reproductive diseases diagnosis and therapy. Int J Mol Sci. https://doi.org/10.3390/ijms22042165
Fan Y, Chen Z, Zhang M (2022) Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J Transl Med 20:291. https://doi.org/10.1186/s12967-022-03493-6
França MM, Funari MFA, Lerario AM, Santos MG, Nishi MY, Domenice S, Moraes DR, Costalonga EF, Maciel GAR, Maciel-Guerra AT, Guerra-Junior G, Mendonca BB (2020) Screening of targeted panel genes in Brazilian patients with primary ovarian insufficiency. PLoS ONE 15:e0240795. https://doi.org/10.1371/journal.pone.0240795
Friedländer MR, Mackowiak SD, Li N, Chen W, Rajewsky N (2012) miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res 40:37–52. https://doi.org/10.1093/nar/gkr688
Gasic S, Mihola O, Trachtulec Z (2022) Prdm9 deficiency of rat oocytes causes synapsis among non-homologous chromosomes and aneuploidy. Mamm Genome 33:590–605. https://doi.org/10.1007/s00335-022-09954-z
Geng Z, Chen H, Zou G, Yuan L, Liu P, Li B, Zhang K, **g F, Nie X, Liu T, Zhang B (2022) Human amniotic fluid mesenchymal stem cell-derived exosomes inhibit apoptosis in ovarian granulosa cell via miR-369-3p/YAF2/PDCD5/p53 pathway. Oxid Med Cell Longev 2022:3695848. https://doi.org/10.1155/2022/3695848
Haller-Kikkatalo K, Uibo R, Kurg A, Salumets A (2015) The prevalence and phenotypic characteristics of spontaneous premature ovarian failure: a general population registry-based study. Hum Reprod 30:1229–1238. https://doi.org/10.1093/humrep/dev021
He F, Liu Y, Li T, Ma Q, Yongmei Z, He P, **ong C (2022) MicroRNA-146 attenuates lipopolysaccharide induced ovarian dysfunction by inhibiting the TLR4/NF-κB signaling pathway. Bioengineered 13:11611–11623. https://doi.org/10.1080/21655979.2022.2070584
Heddar A, Ogur C, Da Costa S, Braham I, Billaud-Rist L, Findikli N, Beneteau C, Reynaud R, Mahmoud K, Legrand S, Marchand M, Cedrin-Durnerin I, Cantalloube A, Peigne M, Bretault M, Dagher-Hayeck B, Perol S, Droumaguet C, Cavkaytar S, Nicolas-Bonne C, Elloumi H, Khrouf M, Rougier-LeMasle C, Fradin M, Le Boette E, Luigi P, Guerrot AM, Ginglinger E, Zampa A, Fauconnier A, Auger N, Paris F, Brischoux-Boucher E, Cabrol C, Brun A, Guyon L, Berard M, Riviere A, Gruchy N, Odent S, Gilbert-Dussardier B, Isidor B, Piard J, Lambert L, Hamamah S, Guedj AM, Brac de la Perriere A, Fernandez H, Raffin-Sanson ML, Polak M, Letur H, Epelboin S, Plu-Bureau G, Wołczyński S, Hieronimus S, Aittomaki K, Catteau-Jonard S, Misrahi M (2022) Genetic landscape of a large cohort of primary ovarian insufficiency: new genes and pathways and implications for personalized medicine. EBioMedicine 84:104246. https://doi.org/10.1016/j.ebiom.2022.104246
Hewlett M, Mahalingaiah S (2015) Update on primary ovarian insufficiency. Curr Opin Endocrinol Diabetes Obes 22:483–489. https://doi.org/10.1097/med.0000000000000206
Huang B, Lu J, Ding C, Zou Q, Wang W, Li H (2018) Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res Ther 9:216. https://doi.org/10.1186/s13287-018-0953-7
Ibrahim A, Marbán E (2016) Exosomes: fundamental biology and roles in cardiovascular physiology. Annu Rev Physiol 78:67–83. https://doi.org/10.1146/annurev-physiol-021115-104929
** Y, Zhang J, Zhu H, Fan G, Zhou G (2020) Expression profiles of miRNAs in giant cell tumor of bone showed miR-187-5p and miR-1323 can regulate biological functions through inhibiting FRS2. Cancer Med 9:3163–3173. https://doi.org/10.1002/cam4.2853
Kozomara A, Griffiths-Jones S (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 39:D152–D157. https://doi.org/10.1093/nar/gkq1027
Kozomara A, Griffiths-Jones S (2014) miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 42:D68-73. https://doi.org/10.1093/nar/gkt1181
Li Y, Yao N, Gao Y, Wang Y, Bai L, Xu J, Wang H (2021) MiR-1224-5p attenuates polycystic ovary syndrome through inhibiting NOD-like receptor protein 3 inflammasome activation via targeting Forkhead box O 1. Bioengineered 12:8555–8569. https://doi.org/10.1080/21655979.2021.1987125
Ling L, Feng X, Wei T, Wang Y, Wang Y, Wang Z, Tang D, Luo Y, **ong Z (2019) Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res Ther 10:46. https://doi.org/10.1186/s13287-019-1136-x
Liu T, Liu Y, Huang Y, Chen J, Yu Z, Chen C, Lai L (2019) miR-15b induces premature ovarian failure in mice via inhibition of α-Klotho expression in ovarian granulosa cells. Free Radic Biol Med 141:383–392. https://doi.org/10.1016/j.freeradbiomed.2019.07.010
Liu T, Lin J, Chen C, Nie X, Dou F, Chen J, Wang Z, Gong Z (2021) MicroRNA-146b-5p overexpression attenuates premature ovarian failure in mice by inhibiting the Dab2ip/Ask1/p38-Mapk pathway and γH2A.X phosphorylation. Cell Prolif 54:e12954. https://doi.org/10.1111/cpr.12954
Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N (2012) How do chemotherapeutic agents damage the ovary? Hum Reprod Update 18:525–535. https://doi.org/10.1093/humupd/dms022
Nelson LM (2009) Clinical practice. Primary ovarian insufficiency. N Engl J Med 360:606–614. https://doi.org/10.1056/NEJMcp0808697
Patiño LC, Silgado D, Laissue P (2017) A potential functional association between mutant BMPR2 and primary ovarian insufficiency. Syst Biol Reprod Med 63:145–149. https://doi.org/10.1080/19396368.2017.1291767
Shamilova NN, Marchenko LA, Dolgushina NV, Zaletaev DV, Sukhikh GT (2013) The role of genetic and autoimmune factors in premature ovarian failure. J Assist Reprod Genet 30:617–622. https://doi.org/10.1007/s10815-013-9974-4
Sun B, Ma Y, Wang F, Hu L, Sun Y (2019) miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther 10:360. https://doi.org/10.1186/s13287-019-1442-3
Toro AU, Shukla SK, Bansal P (2022) Micronome revealed miR-205-5p as key regulator of VEGFA during cancer related angiogenesis in hepatocellular carcinoma. Mol Biotechnol. https://doi.org/10.1007/s12033-022-00619-5
Wang S, Lin S, Zhu M, Li C, Chen S, Pu L, Lin J, Cao L, Zhang Y (2019) Acupuncture reduces apoptosis of granulosa cells in rats with premature ovarian failure via restoring the PI3K/Akt signaling pathway. Int J Mol Sci. https://doi.org/10.3390/ijms20246311
Wang J, Hu Y, Ye C, Liu J (2020) miR-1224-5p inhibits the proliferation and invasion of ovarian cancer via targeting SND1. Hum Cell 33:780–789. https://doi.org/10.1007/s13577-020-00364-4
**ong H, Li Q, Liu S, Wang F, **ong Z, Chen J, Chen H, Yang Y, Tan X, Luo Q, Peng J, **ao G, Jiang Q (2014) Integrated microRNA and mRNA transcriptome sequencing reveals the potential roles of miRNAs in stage I endometrioid endometrial carcinoma. PLoS ONE 9:e110163. https://doi.org/10.1371/journal.pone.0110163
Yang M, Lin L, Sha C, Li T, Zhao D, Wei H, Chen Q, Liu Y, Chen X, Xu W, Li Y, Zhu X (2020) Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab Invest 100:342–352. https://doi.org/10.1038/s41374-019-0321-y
Yang R, Wang D, Han S, Gu Y, Li Z, Deng L, Yin A, Gao Y, Li X, Yu Y, Wang X (2022) MiR-206 suppresses the deterioration of intrahepatic cholangiocarcinoma and promotes sensitivity to chemotherapy by inhibiting interactions with stromal CAFs. Int J Biol Sci 18:43–64. https://doi.org/10.7150/ijbs.62602
Ye X, Pan W, Li C, Ma X, Yin S, Zhou J, Liu J (2020) Exposure to polycyclic aromatic hydrocarbons and risk for premature ovarian failure and reproductive hormones imbalance. J Environ Sci (china) 91:1–9. https://doi.org/10.1016/j.jes.2019.12.015
Yu G, Wang LG, Han Y, He QY (2012) clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16:284–287. https://doi.org/10.1089/omi.2011.0118
Zhang L, Yu D (2019) Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer 1871:455–468. https://doi.org/10.1016/j.bbcan.2019.04.004
Zhang Q, Bu S, Sun J, Xu M, Yao X, He K, Lai D (2017) Paracrine effects of human amniotic epithelial cells protect against chemotherapy-induced ovarian damage. Stem Cell Res Ther 8:270. https://doi.org/10.1186/s13287-017-0721-0
Zhang C, Shen J, Kong S, Zhang M, Zhang Q, Zhou J, Zhen X, Kang N, Jiang Y, Ding L, Sun H, Yan G (2019) MicroRNA-181a promotes follicular granulosa cell apoptosis via sphingosine-1-phosphate receptor 1 expression downregulation†. Biol Reprod 101:975–985. https://doi.org/10.1093/biolre/ioz135
Zhang Y, Han D, Yu X, Shao X, Zong C, Zhang M, Wang J, Liang J, Ge P (2022) MiRNA-190a-5p promotes primordial follicle hyperactivation by targeting PHLPP1 in premature ovarian failure. Front Genet 13:1034832. https://doi.org/10.3389/fgene.2022.1034832
Zheng S, Ma M, Chen Y, Li M (2022) Effects of quercetin on ovarian function and regulation of the ovarian PI3K/Akt/FoxO3a signalling pathway and oxidative stress in a rat model of cyclophosphamide-induced premature ovarian failure. Basic Clin Pharmacol Toxicol 130:240–253. https://doi.org/10.1111/bcpt.13696
Zhou L, **e Y, Li S, Liang Y, Qiu Q, Lin H, Zhang Q (2017) Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovarian Res 10:56. https://doi.org/10.1186/s13048-017-0350-3
Acknowledgements
We acknowledge the GEO researchers for sharing the data.
Funding
This work was supported by Guangdong Basic and Applied Basic Research Foundation (2022A1515111203, 2022A1515012621, 2020B1515120009), National Key S&T Special Projects (2021YFC100530, 2022YFC2703303), National Natural Science Foundation of China (32170617, 31970558), and Foshan Science and Technology project (2020001003953).
Author information
Authors and Affiliations
Contributions
JQL analyzed the data and wrote the manuscript. ZHW assisted with the data analysis. YCZ and ZRS performed the experiment. ZZG and SFM collected the samples. YHL and FX provided ideas and reviewed the manuscript. All the authors contributed to this article and determined the final version.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare no conflicts of interest.
Ethical approval and consent to participate
Approval for the recruitment of participants and sample collection in our research was obtained from the institutional ethics review board of Dongguan Maternal and Child Health Care Hospital and consent to participate was signed by the participants.
Consent for publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1
Hsa-miR-19b-3p inhibited the expression of BMPR2. (a) qPCR analysis of BMPR2 expression in miRNA negative control (NC mimics) and hsa-miR-19b-3p mimics (miR-19b mimics) group in HEK-293T cells. (c) The relative luciferase activity in NC mimics and miR-19b mimics group in HEK-293T cells. *p < 0.05.***p < 0.001. Supplementary file1 (TIF 339 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, J., Wu, Z., Zheng, Y. et al. Plasma-derived exosomal miRNA profiles reveal potential epigenetic pathogenesis of premature ovarian failure. Hum. Genet. (2023). https://doi.org/10.1007/s00439-023-02618-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00439-023-02618-1