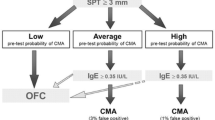
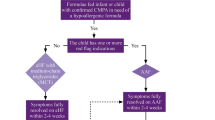
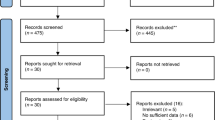
Abstract
The objective of this study was to assess the quality and consistency of recommendations in clinical practice guidelines (CPGs) and expert consensus on paediatric cow’s milk protein allergy (CMPA) to serve as a foundation for future revisions and enhancements of clinical guidelines and consensus documents. We conducted a comprehensive literature search across several databases, including the Chinese Biomedical Literature Database (CBM), PubMed, Embase, Web of Science, UpToDate, ClinicalKey, DynaMed Plus and BMJ Best Practice. We spanned the search period from the inception of each database through October 1, 2023. We integrated subject headings (MeSH/Emtree) and keywords into the search strategy, used the search methodologies of existing literature and developed it in collaboration with a librarian. Two trained researchers independently conducted the literature screening and data extraction. We evaluated methodological quality and recommendations by using the Appraisal of Guidelines for Research & Evaluation II (AGREE II) and AGREE-Recommendations for Excellence (AGREE-REX) tools. Moreover, we compared and summarized key recommendations from high-quality CPGs. Our study included 27 CPGs and expert consensus documents on CMPA. Only four CPGs (14.8%) achieved a high-quality AGREE II rating. The four high-quality CPGs consistently provided recommendations for CMPA. The highest scoring domains for AGREE II were ‘scope and purpose’ (77 ± 12%) and ‘clarity of presentation’ (75 ± 22%). The lowest scoring domains were ‘stakeholder involvement’ (49 ± 21%), ‘rigor of development’ (34 ± 20%) and ‘applicability’ (12 ± 20%). Evaluation with AGREE-REX generally demonstrated low scores across its domains.
Conclusion: Recommendations within high-quality CPGs for the paediatric CMPA showed fundamental consistency. Nevertheless, the methodology and recommendation content of CPGs and the expert consensus exhibited low quality, thus indicating a substantial scope for enhancement. Guideline developers should rigorously follow the AGREE II and AGREE-REX standards in creating CPGs or expert consensuses to guarantee their clinical efficacy in managing paediatric CMPA.
What is Known: • The quality of clinical practice guidelines and expert consensus on paediatric cow's milk protein allergy (CMPA) remains uncertain. • There is a lack of clarity regarding the consistency of crucial recommendations for CMPA management. | |
What is New: • Improving the methodological quality of guidelines and consensus on CMPA requires greater emphasis on stakeholder engagement, rigorous development processes, and practical applicability. • The recommendations from four high-quality guidelines align. However, addressing clinical applicability, integrating values and preferences, and ensuring actionable implementation are critical to improving the quality of all guidelines. |
Graphical Abstract




Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- AGREE II:
-
The Appraisal of Guidelines for Research & Evaluation II
- AGREE-REX:
-
The AGREE-Recommendation for Excellence
- CPG:
-
Clinical practice guidelines
- CMPA:
-
Cow’s milk protein allergy
- CBM:
-
China Biomedical Literature Database
- GRADE:
-
The Grading of Recommendations Assessment, Development, and Evaluation
- ICC:
-
Intraclass correlation coefficient
- IgE:
-
Immunoglobulin E
- OFC:
-
Oral food challenge
- OIT:
-
Oral immunotherapy
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- SPT:
-
Skin prick test
- SIGN:
-
Scottish Intercollegiate Guidelines Network
- WAO:
-
World Allergy Organization
References
Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E (2011) Clinical practice guidelines we can trust. National Academies Press (US) 2011, Washington (DC)
Ruszczyński M, Horvath A, Dziechciarz P, Szajewska H (2016) Cow’s milk allergy guidelines: a quality appraisal with the AGREE II instrument. Clin Exp Allergy: Journal of the British Society for Allergy and Clinical Immunology 46:1236–1241. https://doi.org/10.1111/cea.12784
Zheng Q, Gao Y, **ong L, Huang H, Li J, OuYang G, Saimire W, Yang J, Zhang Y, Wang X, Luo X (2022) Chinese herbal medicine and COVID-19: quality evaluation of clinical guidelines and expert consensus and analysis of key recommendations. Acupuncture and Herbal Medicine 2:152–161. https://doi.org/10.1097/hm9.0000000000000043
Dans AL, Dans LF (2010) Appraising a tool for guideline appraisal the AGREE II instrument. J Clin Epidemiol 63:1281–1282. https://doi.org/10.1016/j.jclinepi.2010.06.005
Luo X, Liu Y, Ren M, Zhang X, Janne E, Lv M, Wang Q, Song Y, Mathew JL, Ahn HS, Lee MS, Chen Y (2021) Consistency of recommendations and methodological quality of guidelines for the diagnosis and treatment of COVID-19. J Evid Based Med 14:40–55. https://doi.org/10.1111/jebm.12419
Norris SL, Louis H, Sawin VI, Porgo TV, Lau YHA, Wang Q, Ferri M (2019) An evaluation of WHO emergency guidelines for Zika virus disease. J Evid Based Med 12:218–224. https://doi.org/10.1111/jebm.12347
Yin Leung AS, Tham EH, Samuel M, Munblit D, Chu DK, Dahdah L, Yamamoto-Hanada K, Trikamjee T, Warad V, van Niekerk A, Martinez S, Ellis A, Bielory L, Cuadros G, van Bever H, Wallace D, Tang M, Sublett J, Wong GWK (2022) Quality and consistency of clinical practice guidelines on the prevention of food allergy and atopic dermatitis: systematic review protocol. World Allergy Organ J https://doi.org/10.1016/j.waojou.2022.100679
AGREE-REX Research Team (2019) The Appraisal of Guidelines Research & Evaluation-Recommendation EXcellence AGREE-REX. https://www.agreetrust.org/wp-content/uploads/2019/04/AGREE-REX-2019.pdf. Accessed 10 Apr 2022
Toca MC, Morais MB, Vázquez-Frias R, Becker-Cuevas DJ, Boggio-Marzet CG, Delgado-Carbajal L, Higuera-Carrillo MM, Ladino L, Marchisone S, Messere GC, Ortiz GJ, Ortiz-Paranza LR, Ortiz-Piedrahita C, Riveros-López JP, Sosa PC, Villalobos-Palencia NC (2022) Consensus on the diagnosis and treatment of cow’s milk protein allergy of the Latin American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Revista de gastroenterologia de Mexico (English) 87:235–250. https://doi.org/10.1016/j.rgmxen.2022.01.002
Meyer R, Venter C, Bognanni A, Szajewska H, Shamir R, Nowak-Wegrzyn A, Fiocchi A, Vandenplas Y (2023) World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) guideline update - VII - milk elimination and reintroduction in the diagnostic process of cow’s milk allergy. World Allergy Organ J. https://doi.org/10.1016/j.waojou.2023.100785
Luyt D, Ball H, Makwana N, Green MR, Bravin K, Nasser SM, Clark AT (2014) BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy : Journal of the British Society for Allergy and Clinical Immunology 44:642–672. https://doi.org/10.1111/cea.12302
Matthai J, Sathiasekharan M, Poddar U, Sibal A, Srivastava A, Waikar Y, Malik R, Ray G, Geetha S, Yachha SK (2020) Guidelines on diagnosis and management of cow’s milk protein allergy. Indian Pediatr 57:723–729
Guler N, Cokugras FC, Sapan N, Selimoglu A, Turktas I, Cokugras H, Aydogan M, Beser OF (2020) Diagnosis and management of cow’s milk protein allergy in Turkey: region-specific recommendations by an expert-panel. Allergologia et Immunopathologia 48:202–210. https://doi.org/10.1016/j.aller.2019.05.004
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L (2010) AGREE II: advancing guideline development, reporting and evaluation in health care. J Clin Epidemiol 63:1308–1311. https://doi.org/10.1016/j.jclinepi.2010.07.001
Brozek JL, Firmino RT, Bognanni A, Arasi S, Ansotegui I, Assa’ad AH, Bahna SL, Canani RB, Bozzola M, Chu DK et al (2022) World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) guideline update - XIV - recommendations on CMA immunotherapy. World Allergy Organ J. https://doi.org/10.1016/j.waojou.2022.100646
Muraro A, de Silva D, Halken S, Worm M, Khaleva E, Arasi S, Dunn-Galvin A, Nwaru BI, De Jong NW, Rodríguez Del Río P et al (2022) Managing food allergy: GA(2)LEN guideline 2022. World Allergy Organ J. https://doi.org/10.1016/j.waojou.2022.100687
Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, Cardona V, Dubois A, Dutoit G, Eigenmann P (2014) EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy 69:1008–1025. https://doi.org/10.1111/all.12429
Alonso-Lebrero E, Bento L, Martorell-Aragonés A, Ribeiro L (2018) Iberian consensus on cow’s milk allergy: the CIBAL Study. Allergologia et immunopathologia 46:517–532. https://doi.org/10.1016/j.aller.2017.10.003
Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH et al (2010) Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 126:S1–58. https://doi.org/10.1016/j.jaci.2010.10.007
Chen T, Hong L, Wang H, Shao J, Yang F, Wang Y, Liu G, Xu X, Sheng X, Xu C (2022) Guidelines for diagnosis and nutritional intervention of mild to moderate non-IgE mediated cow’s milk protein allergy in Chinese infants. Chin J Appl Clin Pediatr 37:241–250. https://doi.org/10.3760/cma.j.cn101070-20220106-00016
El-Hodhod MA, El-Shabrawi MHF, AlBadi A, Hussein A, Almehaidib A, Nasrallah B, AlBassam EM, El Feghali H, Isa HM, Al Saraf K, Sokhn M, Adeli M, Al-Sawi NMM, Hage P, Al-Hammadi S (2021) Consensus statement on the epidemiology, diagnosis, prevention, and management of cow’s milk protein allergy in the Middle East: a modified Delphi-based study. World journal of pediatrics : WJP 17:576–589. https://doi.org/10.1007/s12519-021-00476-3
Kemp AS, Hill DJ, Allen KJ, Anderson K, Davidson GP, Day AS, Heine RG, Peake JE, Prescott SL, Shugg AW, Sinn JK (2008) Guidelines for the use of infant formulas to treat cows milk protein allergy: an Australian consensus panel opinion. Med J Aust 188:109–112. https://doi.org/10.5694/j.1326-5377.2008.tb01534.x
Ribes-Koninckx C, Amil-Dias J, Espin B, Molina M, Segarra O, Diaz-Martin JJ (2023) The use of amino acid formulas in pediatric patients with allergy to cow’s milk proteins: recommendations from a group of experts. Front Pediatr. https://doi.org/10.3389/fped.2023.1110380
Vandenplas Y, Al-Hussaini B, Al-Mannaei K, Al-Sunaid A, Ayesh WH, El-Degeir M, El-Kabbany N, Haddad J, Hashmi A, Kreishan F, Tawfik E (2019) Prevention of allergic sensitization and treatment of cow’s milk protein allergy in early life: the middle-east step-down consensus. Nutrients. https://doi.org/10.3390/nu11071444
Allen KJ, Davidson GP, Day AS, Hill DJ, Kemp AS, Peake JE, Prescott SL, Shugg A, Sinn JK, Heine RG (2009) Management of cow’s milk protein allergy in infants and young children: an expert panel perspective. J Paediatr Child Health 45:481–486. https://doi.org/10.1111/j.1440-1754.2009.01546.x
Caffarelli C, Baldi F, Bendandi B, Calzone L, Marani M, Pasquinelli P, Ewgpag (2010) Cow’s milk protein allergy in children: a practical guide. Ital J Pediatr. https://doi.org/10.1186/1824-7288-36-5
Dupont C, Chouraqui JP, de Boissieu D, Bocquet A, Bresson JL, Briend A, Darmaun D, Frelut ML, Ghisolfi J, Girardet JP, Goulet O, Hankard R, Rieu D, Vidailhet M, Turck D (2012) Dietary treatment of cows’ milk protein allergy in childhood: a commentary by the Committee on Nutrition of the French Society of Paediatrics. Br J Nutr 107:325–338. https://doi.org/10.1017/s0007114511004831
Espín Jaime B, Díaz Martín JJ, Blesa Baviera LC, Claver Monzón Á, Hernández Hernández A, García Burriel JI, Mérida MJG, Pinto Fernández C, Coronel Rodríguez C, Román Riechmann E, Ribes Koninckx C (2019) Non-IgE-mediated cow’s milk allergy: Consensus document of the Spanish Society of Paediatric Gastroenterology, Hepatology, and Nutrition (SEGHNP), the Spanish Association of Paediatric Primary Care (AEPAP), the Spanish Society of Extra-hospital Paediatrics and Primary Health Care (SEPEAP), and the Spanish Society of Paediatric ClinicaL Immunology, Allergy, and Asthma (SEICAP). Anales de pediatria 90:193.e191–193. https://doi.org/10.1016/j.anpedi.2018.11.007
Kansu A, Yuce A, Dalgic B, Sekerel BE, Cullu-Cokugras F, Cokugras H (2016) Consensus statement on diagnosis, treatment and follow-up of cow’s milk protein allergy among infants and children in Turkey. Turk J Pediatr 58:1–11. https://doi.org/10.24953/turkjped.2016.01.001
Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, Staiano A, Schäppi MG, Vandenplas Y (2012) Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr 55:221–229. https://doi.org/10.1097/MPG.0b013e31825c9482
Vandenplas Y, Abuabat A, Al-Hammadi S, Aly GS, Miqdady MS, Shaaban SY, Torbey PH (2014) Middle East Consensus Statement on the Prevention, Diagnosis, and Management of Cow’s Milk Protein Allergy. Pediat Gastroenterol Hepatol Nutr 17:61–73. https://doi.org/10.5223/pghn.2014.17.2.61
Venter C, Brown T, Shah N, Walsh J, Fox AT (2013) Diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy - a UK primary care practical guide. Clin Transl Allergy. https://doi.org/10.1186/2045-7022-3-23
Fleischer DM, Chan ES, Venter C, Spergel JM, Abrams EM, Stukus D, Groetch M, Shaker M, Greenhawt M (2021) A consensus approach to the primary prevention of food allergy through nutrition: guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; and the Canadian Society for Allergy and Clinical Immunology. J Allergy Clin Immunol Pract 9:22–43.e24. https://doi.org/10.1016/j.jaip.2020.11.002
Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, Nadeau K, Nowak-Wegrzyn A, Oppenheimer J, Perry TT et al (2014) Food allergy: a practice parameter update-2014. J Allergy Clin Immunol 134:1016–1025.e1043. https://doi.org/10.1016/j.jaci.2014.05.013
ASACI (2022) Guide for milk substitutes in cow’s milk allergy
Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, Nadeau K, Nowak-Wegrzyn A, Oppenheimer J, Perry TT, Randolph C, Sicherer SH, Simon RA, Vickery BP, Wood R (2014) Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2014.05.013
Solà I, Carrasco JM, Díaz Del Campo P, Gracia J, Orrego C, Martínez F, Kotzeva A, Guillamón I, Calderón E, de Gaminde I, Louro A, Rotaeche R, Salcedo F, Velázquez P, Alonso-Coello P (2014) Attitudes and perceptions about clinical guidelines: a qualitative study with Spanish physicians. PloS one. https://doi.org/10.1371/journal.pone.0086065
Yao-long C, Ke-hu Y, **-hui T, Qaseem A (2016) Development of evidence-based practice guidelines: international experience and Chinese practice. Journal of Lanzhou University Medical Sciences 42:29–35. https://doi.org/10.13885/j.issn.1000-2812.2016.01.006
El Hajj MS, Jaam M, Sheikh Ali SAS, Saleh R, Awaisu A, Paravattil B, Wilby KJ (2021) Critical appraisal of tobacco dependence treatment guidelines. Int J Clin Pharm 43:85–100. https://doi.org/10.1007/s11096-020-01110-4
García LM, Sanabria AJ, Alvarez EG, Trujillo-Martín MM, Etxeandia-Ikobaltzeta I, Kotzeva A, Rigau D, Louro-González A, Barajas-Nava L, Del Campo PD, Estrada MD, Solà I, Gracia J, Salcedo-Fernandez F, Lawson J, Haynes RB, Alonso-Coello P (2014) The validity of recommendations from clinical guidelines: a survival analysis. CMAJ : Canadian Medical Association Journal = Journal de l’Association medicale canadienne 186:1211–1219. https://doi.org/10.1503/cmaj.140547
Hill JE, Stephani AM, Sapple P, Clegg AJ (2020) The effectiveness of continuous quality improvement for develo** professional practice and improving health care outcomes: a systematic review. Implementation Science. https://doi.org/10.1186/s13012-020-0975-2
Horbar JD, Carpenter JH, Buzas J, Soll RF, Suresh G, Bracken MB, Leviton LC, Plsek PE, Sinclair JC (2004) Collaborative quality improvement to promote evidence based surfactant for preterm infants: a cluster randomised trial. BMJ (Clinical research ed). https://doi.org/10.1136/bmj.329.7473.1004
Innvaer S, Vist G, Trommald M, Oxman A (2002) Health policy-makers’ perceptions of their use of evidence: a systematic review. J Health Serv Res Policy 7:239–244. https://doi.org/10.1258/135581902320432778
Langlois EV, Becerril Montekio V, Young T, Song K, Alcalde-Rabanal J, Tran N (2016) Enhancing evidence informed policymaking in complex health systems: lessons from multisite collaborative approaches. Health Res Policy Syst. https://doi.org/10.1186/s12961-016-0089-0
Francke AL, Smit MC, de Veer AJ, Mistiaen P (2008) Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. https://doi.org/10.1186/1472-6947-8-38
Tricco AC, Zarin W, Rios P, Nincic V, Khan PA, Ghassemi M, Diaz S, Pham B, Straus SE, Langlois EV (2018) Engaging policy-makers, health system managers, and policy analysts in the knowledge synthesis process: a sco** review. Implementation science. https://doi.org/10.1186/s13012-018-0717-x
Sun Y, Gao Y, Chen J, Sun H, Cai YT, Ge L, Li YN, Zhang J, Tian JH (2019) Evidence map** of recommendations on diagnosis and therapeutic strategies for diabetes foot: an international review of 22 guidelines. Metab Clin Exp. https://doi.org/10.1016/j.metabol.2019.153956
D’Auria E, Venter C (2020) Precision medicine in cow’s milk allergy. Curr Opin Allergy Clin Immunol 20:233–241. https://doi.org/10.1097/aci.0000000000000640
Acknowledgements
We extend our deepest gratitude to the dedicated individuals who developed the guidelines, the insightful reviewers for their invaluable feedback, our research team for their unwavering support and collaboration throughout this study, and express our sincere appreciation for the support provided by the Natural Science Foundation of Gansu Province. This work was supported by the Natural Science Foundation of Gansu Province (21JR11RA178).
Author information
Authors and Affiliations
Contributions
Tengfei Li and **hui Tian conceived the manuscript. Tengfei Li and Qingyong Zheng drafted the manuscript. Mingyue Zhang, Yiyi Li, and Yongjia Zhou filtered the articles. Mingyue Zhang, Yiyi Li, Yongjia Zhou, Caihua Xu, Bowa Zhang, and Zeiwei Wang analysed the data and constructed the graphs. **hui Tian and Li Zhou provided a critical version of the manuscript. All authors contributed to the revision of the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Gregorio Milani
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, T., Zheng, Q., Zhang, M. et al. How consistent are the key recommendations, and what is the quality of guidelines and expert consensus regarding paediatric cow’s milk protein allergy?. Eur J Pediatr (2024). https://doi.org/10.1007/s00431-024-05622-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00431-024-05622-3