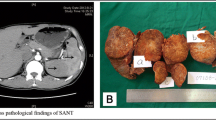
Abstract
Sclerosing angiomatoid nodular transformation (SANT) is a rare vascular lesion of the spleen. Although several hypotheses have been suggested, the etiopathogenesis of SANT remains unknown. It is also unclear whether SANT is a reactive or a neoplastic lesion. Since CTNNB1 (β-catenin gene) exon 3 mutations were frequently detected in some rare fibrovascular lesions, we aimed to investigate the presence of oncogenic CTNNB1 mutations in SANT cases. For this purpose, 7 cases of SANT with typical histopathological features were retrieved. First, the presence of CTNNB1 exon 3 alterations was examined with a recently described immunohistochemistry-based method. Then, the findings were confirmed with polymerase chain reaction (PCR), reverse transcription PCR (RT-PCR), and Sanger sequencing. In all cases, immunochemistry of β-catenin gave a staining pattern that was suggestive of exon 3 alteration; however, no missense mutations were found in any case at the CTNNB1 exon 3 hotspot region. Subsequently, we screened for large interstitial deletions of CTNNB1 exon 3 which revealed short PCR products in three cases. Sequencing confirmed that these cases had large interstitial deletions, resulting in loss of the entire exon 3 of CTNNB1. In the remaining four cases, loss of exon 3 was documented at the cDNA level, although genomic deletion was not identified. These results demonstrate that loss of CTNNB1 exon 3 and stabilization of β-catenin with activation of Wnt signaling pathway might have a significant role in the pathogenesis of SANT. Through this study, we provided important evidence for the neoplastic nature and pathogenesis of this disorder.


Similar content being viewed by others
Data and material availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
Code availability
Not applicable.
References
Martel M, Cheuk W, Lombardi L, Lifschitz-Mercer B, Chan JKC, Rosai J (2004) Sclerosing angiomatoid nodular transformation (SANT): report of 25 cases of a distinctive benign splenic lesion. Am J Surg Pathol 28:1268–1279. https://doi.org/10.1097/01.pas.0000138004.54274.d3
Weinreb I, Bailey D, Battaglia D, Kennedy M, Perez-Ordoñez B (2007) CD30 and Epstein–Barr virus RNA expression in sclerosing angiomatoid nodular transformation of spleen. Virchows Arch 451:73–79. https://doi.org/10.1007/s00428-007-0422-7
Kashiwagi S, Kumasaka T, Bunsei N, Fukumura Y, Yamasaki S, Abe K, Mitani K, Abe H, Matsumoto T, Suda K (2008) Detection of Epstein–Barr virus-encoded small RNA-expressed myofibroblasts and IgG4-producing plasma cells in sclerosing angiomatoid nodular transformation of the spleen. Virchows Arch 453:275–282. https://doi.org/10.1007/s00428-008-0648-z
Nagai Y, Hayama N, Kishimoto T, Furuya M, Takahashi Y, Otsuka M, Miyazaki M, Nakatani Y (2008) Predominance of IgG4+ plasma cells and CD68 positivity in sclerosing angiomatoid nodular transformation (SANT). Histopathology 53:495–498. https://doi.org/10.1111/j.1365-2559.2008.03118.x
Kuo T, Chen T-C, Lee L (2009) Sclerosing angiomatoid nodular transformation of the spleen (SANT): clinicopathological study of 10 cases with or without abdominal disseminated calcifying fibrous tumors, and the presence of a significant number of IgG4+ plasma cells. Pathol Int 59:844–850. https://doi.org/10.1111/j.1440-1827.2009.02456.x
Pradhan D, Mohanty SK (2013) Sclerosing angiomatoid nodular transformation of the spleen. Arch Pathol Lab Med 137:1309–1312. https://doi.org/10.5858/arpa.2012-0601-RS
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14:1837–1851
Abraham SC, Montgomery EA, Giardiello FM, Wu T-T (2001) Frequent β-catenin mutations in juvenile nasopharyngeal angiofibromas. Am J Pathol 158:1073–1078. https://doi.org/10.1016/S0002-9440(10)64054-0
Haller F, Bieg M, Moskalev EA, Barthelmeß S, Geddert H, Boltze C, Diessl N, Braumandl K, Brors B, Iro H, Hartmann A, Wiemann S, Agaimy A (2015) Recurrent mutations within the amino-terminal region of β-catenin are probable key molecular driver events in sinonasal hemangiopericytoma. Am J Pathol 185:563–571. https://doi.org/10.1016/j.ajpath.2014.10.019
Jung S-H, Kim MS, Lee S-H, Park HC, Choi HJ, Maeng L, Min KO, Kim J, Park TI, Shin OR, Kim TJ, Xu H, Lee KY, Kim TM, Song SY, Lee C, Chung YJ, Lee SH (2016) Whole-exome sequencing identifies recurrent AKT1 mutations in sclerosing hemangioma of lung. Proc Natl Acad Sci 113:10672–10677. https://doi.org/10.1073/pnas.1606946113
Guo Y, **ao L, Sun L, Liu F (2012) Wnt/β-catenin signaling: a promising new target for fibrosis diseases. Physiol Res:337–346. https://doi.org/10.33549/physiolres.932289
Akyol A, Güner G, Özşeker HS, Işık A, Atcı Ö, Uzun S, Atayar E, Ozaltin F, Gedikoğlu G, Sökmensüer C, Fearon ER (2018) An immunohistochemical approach to detect oncogenic CTNNB1 mutations in primary neoplastic tissues. Lab Investig 99:128–137. https://doi.org/10.1038/s41374-018-0121-9
Dubbink HJ, Hollink IHIM, Avenca Valente C, Wang W, Liu P, Doukas M, van Noesel MM, Dinjens WNM, Wagner A, Smits R (2018) A novel tissue-based ß-catenin gene and immunohistochemical analysis to exclude familial adenomatous polyposis among children with hepatoblastoma tumors. Pediatr Blood Cancer 65:e26991. https://doi.org/10.1002/pbc.26991
Banerjee SK, Makdisi WF, Weston AP et al (1995) Microwave-based DNA extraction from paraffin-embedded tissue for PCR amplification. BioTechniques 18(768–770):772–773
Gao C, Wang Y, Broaddus R et al (2018) Exon 3 mutations of CTNNB1 drive tumorigenesis: a review. Oncotarget 9:5492–5508. https://doi.org/10.18632/oncotarget.23695
Agaimy A, Haller F (2016) CTNNB1 (β-catenin)-altered neoplasia: a review focusing on soft tissue neoplasms and parenchymal lesions of uncertain histogenesis. Adv Anat Pathol 23:1–12. https://doi.org/10.1097/PAP.0000000000000104
Dômont J, Salas S, Lacroix L, Brouste V, Saulnier P, Terrier P, Ranchère D, Neuville A, Leroux A, Guillou L, Sciot R, Collin F, Dufresne A, Blay JY, le Cesne A, Coindre JM, Bonvalot S, Bénard J (2010) High frequency of β-catenin heterozygous mutations in extra-abdominal fibromatosis: a potential molecular tool for disease management. Br J Cancer 102:1032–1036. https://doi.org/10.1038/sj.bjc.6605557
Lasota J, Felisiak-Golabek A, Aly FZ, Wang ZF, Thompson LDR, Miettinen M (2015) Nuclear expression and gain-of-function β-catenin mutation in glomangiopericytoma (sinonasal-type hemangiopericytoma): insight into pathogenesis and a diagnostic marker. Mod Pathol 28:715–720. https://doi.org/10.1038/modpathol.2014.161
Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, Cole CG, Ward S, Dawson E, Ponting L, Stefancsik R, Harsha B, Kok CY, Jia M, Jubb H, Sondka Z, Thompson S, de T, Campbell PJ (2017) COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res 45:D777–D783. https://doi.org/10.1093/nar/gkw1121
Iwao K, Nakamori S, Kameyama M, Imaoka S, Kinoshita M, Fukui T, Ishiguro S, Nakamura Y, Miyoshi Y (1998) Activation of the beta-catenin gene by interstitial deletions involving exon 3 in primary colorectal carcinomas without adenomatous polyposis coli mutations. Cancer Res 58:1021–1026
Liang Y, Feng Y, Zong M, Wei XF, Lee J, Feng Y, Li H, Yang GS, Wu ZJ, Fu XD, Feng GS (2018) β-catenin deficiency in hepatocytes aggravates hepatocarcinogenesis driven by oncogenic β-catenin and MET: LIANG, FENG, ET AL. Hepatology 67:1807–1822. https://doi.org/10.1002/hep.29661
Gilbert MTP, Haselkorn T, Bunce M, Sanchez JJ, Lucas SB, Jewell LD, Marck EV, Worobey M (2007) The isolation of nucleic acids from fixed, paraffin-embedded tissues–which methods are useful when? PLoS ONE 2:e537. https://doi.org/10.1371/journal.pone.0000537
Campos PF, Gilbert TMP (2012) DNA Extraction from formalin-fixed material. In: Shapiro B, Hofreiter M (eds) Ancient DNA. Humana Press, Totowa, NJ, pp 81–85
Pagenstecher C, Wehner M, Friedl W, Rahner N, Aretz S, Friedrichs N, Sengteller M, Henn W, Buettner R, Prop** P, Mangold E (2006) Aberrant splicing in MLH1 and MSH2 due to exonic and intronic variants. Hum Genet 119:9–22. https://doi.org/10.1007/s00439-005-0107-8
Eswaran J, Horvath A, Godbole S, Reddy SD, Mudvari P, Ohshiro K, Cyanam D, Nair S, Fuqua SAW, Polyak K, Florea LD, Kumar R (2013) RNA sequencing of cancer reveals novel splicing alterations. Sci Rep 3:1689. https://doi.org/10.1038/srep01689
Schrock AB, Frampton GM, Suh J, Chalmers ZR, Rosenzweig M, Erlich RL, Halmos B, Goldman J, Forde P, Leuenberger K, Peled N, Kalemkerian GP, Ross JS, Stephens PJ, Miller VA, Ali SM, Ou SHI (2016) Characterization of 298 patients with lung cancer harboring MET exon 14 skip** alterations. J Thorac Oncol 11:1493–1502. https://doi.org/10.1016/j.jtho.2016.06.004
Mou H, Smith JL, Peng L, Yin H, Moore J, Zhang XO, Song CQ, Sheel A, Wu Q, Ozata DM, Li Y, Anderson DG, Emerson CP, Sontheimer EJ, Moore MJ, Weng Z, Xue W (2017) CRISPR/Cas9-mediated genome editing induces exon skip** by alternative splicing or exon deletion. Genome Biol 18:108. https://doi.org/10.1186/s13059-017-1237-8
Chang K-C, Lee J-C, Wang Y-C et al (2016) Polyclonality in sclerosing angiomatoid nodular transformation of the spleen. Am J Surg Pathol 40:1343–1351. https://doi.org/10.1097/PAS.0000000000000716
Tajima S, Koda K (2015) A case of cord capillary hemangioma of the spleen: a recently proven true neoplasm: Splenic cord capillary hemangioma. Pathol Int 65:254–258. https://doi.org/10.1111/pin.12277
Chiu A, Czader M, Cheng L, Hasserjian RP, Wang M, Bhagavathi S, Hyjek EM, al-Ahmadie H, Knowles DM, Orazi A (2011) Clonal X-chromosome inactivation suggests that splenic cord capillary hemangioma is a true neoplasm and not a subtype of splenic hamartoma. Mod Pathol 24:108–116. https://doi.org/10.1038/modpathol.2010.168
Author information
Authors and Affiliations
Contributions
S.U., Ö.Ö., and A.I. carried out the experiments; A.S., G.G., A.S.D., I.K., A.Ü., and A.A. selected the cases and confirmed the diagnosis of SANT; S.U. and A.A. wrote the first draft; all authors interpreted the collected data and critically reviewed the manuscript. The final version of the manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval
The study was executed according to the regulations of the Non-Interventional Clinical Research Ethics Committee of Hacettepe University.
Consent to participate
Not applicable.
Consent for publication
The authors hereby declare that they give their consent for this submitted work to be published in Virchows Archiv European Journal of Pathology.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2851 kb)
Rights and permissions
About this article
Cite this article
Uzun, S., Özcan, Ö., Işık, A. et al. Loss of CTNNB1 exon 3 in sclerosing angiomatoid nodular transformation of the spleen. Virchows Arch 479, 747–754 (2021). https://doi.org/10.1007/s00428-021-03064-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03064-y