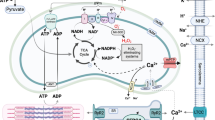
Abstract
TRPM4 is a Ca2+-activated nonselective cation channel involved in cardiovascular physiology and pathophysiology. Based on cellular experiments and numerical simulations, the present study aimed to explore the potential arrhythmogenicity of CaMKII-mediated TRPM4 channel overactivation linked to Ca2+ dysregulation in the heart. The confocal immunofluorescence microscopy, western blot, and proximity ligation assay (PLA) in HL-1 atrial cardiomyocytes and/or TRPM4-expressing TSA201 cells suggested that TRPM4 and CaMKII proteins are closely localized. Co-expression of TRPM4 and CaMKIIδ or a FRET-based sensor Camui in HEK293 cells showed that the extent of TRPM4 channel activation was correlated with that of CaMKII activity, suggesting their functional interaction. Both expressions and interaction of the two proteins were greatly enhanced by angiotensin II treatment, which induced early afterdepolarizations (EADs) at the repolarization phase of action potentials (APs) recorded from HL-1 cells by the current clamp mode of patch clamp technique. This arrhythmic change disappeared after treatment with the TRPM4 channel blocker 9-phenanthrol or CaMKII inhibitor KN-62. In order to quantitatively assess how CaMKII modulates the gating behavior of TRPM4 channel, the ionomycin-permeabilized cell-attached recording was employed to obtain the voltage-dependent parameters such as steady-state open probability and time constants for activation/deactivation at different [Ca2+]i. Numerical simulations incorporating these kinetic data into a modified HL-1 model indicated that > 3-fold increase in TRPM4 current density induces EADs at the late repolarization phase and CaMKII inhibition (by KN-62) completely eliminates them. These results collectively suggest a novel arrhythmogenic mechanism involving excessive CaMKII activity that causes TRPM4 overactivation in the stressed heart.








Similar content being viewed by others
References
Adachi-Akahane S, Cleemann L, Morad M (1996) Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. J Gen Physiol 108:435–454
Ardestani G, West MC, Maresca TJ, Fissore RA, Stratton MM (2019) FRET-based sensor for CaMKII activity (FRESCA): a useful tool for assessing CaMKII activity in response to Ca2+ oscillations in live cells. J Biol Chem 294:11876–11891. https://doi.org/10.1074/jbc.RA119.009235
Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA (2009) The δ isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci 106:2342–2347
Bagur R, Hajnoczky G (2017) Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol Cell 66:780–788. https://doi.org/10.1016/j.molcel.2017.05.028
Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415:198–205. https://doi.org/10.1038/415198a
Claycomb WC, Lanson NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ (1998) HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. P Natl Acad Sci U S A 95:2979–2984. https://doi.org/10.1073/pnas.95.6.2979
Dragún M, Gažová A, Kyselovič J, Hulman M, Máťuš M (2019) TRP channels expression profile in human end-stage heart failure. Medicina 55:380
Duan J, Li Z, Li J, Santa-Cruz A, Sanchez-Martinez S, Zhang J, Clapham DE (2018) Structure of full-length human TRPM4. Proc Natl Acad Sci U S A 115:2377–2382. https://doi.org/10.1073/pnas.1722038115
Gees M, Colsoul B, Nilius B (2010) The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2:a003962. https://doi.org/10.1101/cshperspect.a003962
Glynn P, Musa H, Wu XQ, Unudurthi SD, Little S, Qian L, Wright PJ, Radwanski PB, Gyorke S, Mohler PJ, Hund TJ (2015) Voltage-gated sodium channel phosphorylation at Ser571 regulates late current, arrhythmia, and cardiac function in vivo. Circulation 132:567–577. https://doi.org/10.1161/Circulationaha.114.015218
Guinamard R, Demion M, Magaud C, Potreau D, Bois P (2006) Functional expression of the TRPM4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension 48:587–594. https://doi.org/10.1161/01.HYP.0000237864.65019.a5
Heijman J, Voigt N, Nattel S, Dobrev D (2014) Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 114:1483–1499. https://doi.org/10.1161/CIRCRESAHA.114.302226
Hof T, Simard C, Rouet R, Salle L, Guinamard R (2013) Implication of the TRPM4 nonselective cation channel in mammalian sinus rhythm. Heart Rhythm 10:1683–1689. https://doi.org/10.1016/j.hrthm.2013.08.014
Hof T, Sallé L, Coulbault L, Richer R, Alexandre J, Rouet R, Manrique A, Guinamard R (2016) TRPM4 non-selective cation channels influence action potentials in rabbit Purkinje fibres. J Physiol 594:295–306
Hof T, Chaigne S, Recalde A, Salle L, Brette F, Guinamard R (2019) Transient receptor potential channels in cardiac health and disease. Nat Rev Cardiol 16:344–360. https://doi.org/10.1038/s41569-018-0145-2
Hu YP, Duan YB, Takeuchi A, Hai-Kurahara L, Ichikawa J, Hiraishi K, Numata T, Ohara H, Iribe G, Nakaya M, Mori MX, Matsuoka S, Ma G, Inoue R (2017) Uncovering the arrhythmogenic potential of TRPM4 activation in atrial-derived HL-1 cells using novel recording and numerical approaches. Cardiovasc Res 113:1243–1255. https://doi.org/10.1093/cvr/cvx117
Inoue R, Ito Y (2000) Intracellular ATP slows time-dependent decline of muscarinic cation current in guinea pig ileal smooth muscle. Am J Phys Cell Phys 279:C1307–C1318. https://doi.org/10.1152/ajpcell.2000.279.5.C1307
Jeevaratnam K, Chadda KR, Huang CL, Camm AJ (2018) Cardiac potassium channels: physiological insights for targeted therapy. J Cardiovasc Pharmacol Ther 23:119–129. https://doi.org/10.1177/1074248417729880
Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M, Zhao JJ, Yang XP, Alkan O, Kim S, Harris JL, Wilson CJ, Myer VE, Finan PM, Root DE, Roberts TM, Golub T, Flaherty KT, Dummer R, Weber BL, Sellers WR, Schlegel R, Wargo JA, Hahn WC, Garraway LA (2010) COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468:968–U370. https://doi.org/10.1038/nature09627
Lam AJ, St-Pierre F, Gong Y, Marshall JD, Cranfill PJ, Baird MA, McKeown MR, Wiedenmann J, Davidson MW, Schnitzer MJ, Tsien RY, Lin MZ (2012) Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods 9:1005–1012. https://doi.org/10.1038/nmeth.2171
Luczak ED, Anderson ME (2014) CaMKII oxidative activation and the pathogenesis of cardiac disease. J Mol Cell Cardiol 73:112–116. https://doi.org/10.1016/j.yjmcc.2014.02.004
Luo CH, Rudy Y (1994) A dynamic model of the cardiac ventricular action potential. II. Afterdepolarizations, triggered activity, and potentiation. Circ Res 74:1097–1113. https://doi.org/10.1161/01.res.74.6.1097
Mathar I, Kecskes M, Van der Mieren G, Jacobs G, Camacho Londoño JE, Uhl S, Flockerzi V, Voets T, Freichel M, Nilius B (2014) Increased β-adrenergic inotropy in ventricular myocardium from Trpm4−/− mice. Circ Res 114:283–294
Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX (2005) Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem 280:6423–6433. https://doi.org/10.1074/jbc.M411089200
Sag CM, Mallwitz A, Wagner S, Hartmann N, Schotola H, Fischer TH, Ungeheuer N, Herting J, Shah AM, Maier LS, Sossalla S, Unsold B (2014) Enhanced late INa induces proarrhythmogenic SR Ca leak in a CaMKII-dependent manner. J Mol Cell Cardiol 76:94–105. https://doi.org/10.1016/j.yjmcc.2014.08.016
Shy D, Gillet L, Ogrodnik J, Albesa M, Verkerk AO, Wolswinkel R, Rougier JS, Barc J, Essers MC, Syam N, Marsman RF, van Mil AM, Rotman S, Redon R, Bezzina CR, Remme CA, Abriel H (2014) PDZ domain-binding motif regulates cardiomyocyte compartment-specific NaV1.5 channel expression and function. Circulation 130:147–160. https://doi.org/10.1161/CIRCULATIONAHA.113.007852
Simard C, Sallé L, Rouet R, Guinamard R (2012) Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle. Br J Pharmacol 165:2354–2364
Son MJ, Kim JC, Kim SW, Chidipi B, Muniyandi J, Singh TD, So I, Subedi KP, Woo SH (2016) Shear stress activates monovalent cation channel transient receptor potential melastatin subfamily 4 in rat atrial myocytes via type 2 inositol 1, 4, 5-trisphosphate receptors and Ca2+ release. J Physiol 594:2985–3004
Swaminathan PD, Purohit A, Hund TJ, Anderson ME (2012) Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res 110:1661–1677. https://doi.org/10.1161/CIRCRESAHA.111.243956
Takeuchi A, Kim B, Matsuoka S (2013) The mitochondrial Na+-Ca2+ exchanger, NCLX, regulates automaticity of HL-1 cardiomyocytes. Sci Rep 3:2766
Wang J, Takahashi K, Piao H, Qu P, Naruse K (2013) 9-Phenanthrol, a TRPM4 inhibitor, protects isolated rat hearts from ischemia-reperfusion injury. PLoS One 8:e70587. https://doi.org/10.1371/journal.pone.0070587
Yang Z, Murray KT (2011) Ionic mechanisms of pacemaker activity in spontaneously contracting atrial HL-1 cells. J Cardiovasc Pharmacol 57:28–36. https://doi.org/10.1097/FJC.0b013e3181fda7c4
Availability of data and material (data transparency)
The data described and materials used in this paper are available on request.
Funding
This work has been supported by a JSPS Grant-in-Aid for Young Scientists (B) (No. 17K15566) to Y.H., a JSPS Grant-in-Aid for Scientific Research (B) (No. 15H04678) to R.I., and Swiss National Science Foundation to H.A. (No. 310030_184783).
Author information
Authors and Affiliations
Contributions
Y.H., H.A., and R.I. conceived this study; Y.H. conducted patch clamp experiments and analyzed the obtained data; Y.H., DR.K., M.E., and P.A. contributed immunoblotting, immunofluorescence, and PLA experiments and analyzed the obtained data; R.I. and Y.H. conducted simulations on the HL-1 AP model. T.F. helped to design the work and commented on the draft. Y.H., R.I., and H.A. contributed funding acquisition; Y.H. and R.I. wrote the manuscript and all authors reviewed and commented it.
Corresponding authors
Ethics declarations
Conflict of interest
Not applicable
Ethics approval
Not applicable
Consent to participate
All authors confirmed that their roles played in this work are properly appreciated, and approved the order of authorship. The authors gratefully acknowledge Jean-Sébastien Rougier for constructive comments during experiments.
Consent for publication
All authors agreed to submit/publish this paper after careful reading.
Code availability (software application or custom code)
The codes for modified HL-1 models incorporating TRPM4 kinetics with and without CaMKII inhibition (written in CellML) can be provided on request. The original HL-1 model was written in Visual C++ (Takeuchi et al., 2013) which was translated into CellML (by Hu and Inoue). The Luo-Rudy 2000 model is available from the Cor library (Oxford; URL: http://cor.physiol.ox.ac.uk/).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributions to Special Issues
This article is published as part of the Special Issue on Calcium Signal Dynamics in Cardiac Myocytes and Fibroblasts: Mechanisms and Therapeutics.
This article is part of the special issue on Calcium Signal Dynamics in Cardiac Myocytes and Fibroblasts: Mechanisms in Pflügers Archiv—European Journal of Physiology
Supplementary information
Supplementary Fig. 1
Reconstructed rate constants and open probability for TRPM4 channel gating in the absence and presence of KN-62. The rate constants of opening (α) and closing (β) are calculated with respect to two variables, i.e. membrane potential (Vm) and intracellular Ca2+ concentration ([Ca2+]i), by using the equations in the main text. Open probability (Po) is then calculated by the formula: α/(α+β). In the absence of KN-62 (con), α and β show reciprocal voltage-dependency over a wide range of [Ca2+]i. These rate constants however become much less sensitive to Vm after CaMKII inhibition by KN-62 (KN62), making α and β stay at low and high values, respectively. As a result, (except for extremely high [Ca2+]i) the transition from the ‘O’ to ‘C’ states is decelerated, while that from the ‘O’ to ‘C’ states is accelerated by CaMKII inhibition to favor the ‘C’ state, which renders the deactivation of TRPM4 channel faster around the resting membrane potential. Accordingly, Po at given Vm and [Ca2+]i becomes smaller and less voltage-dependent. Supplementary Fig. 2 AP simulation with Luo-Rudy 2000 model. Luo-Rudy AP 2000 models with TRPM4 gating kinetics before and after application of KN-62. The density of TRPM4 channel is increased from normal (see the Methods) to several-fold levels. (PPTX 282 kb)
Rights and permissions
About this article
Cite this article
Hu, Y., Kaschitza, D.R., Essers, M. et al. Pathological activation of CaMKII induces arrhythmogenicity through TRPM4 overactivation. Pflugers Arch - Eur J Physiol 473, 507–519 (2021). https://doi.org/10.1007/s00424-020-02507-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02507-w