Abstract
Purpose
Fluorescein angiography (FA) has been conventionally used for detection of retinal nonperfused area (NPA) in diabetic retinopathy (DR) in spite of its qualitative evaluation. Optical coherence tomography angiography (OCTA) has been recently reported to be useful for the quantification of retinal vascular disorder in DR. In this study, we examined whether retinal flow density (FD) measurement in OCTA was useful for NPA detection in DR.
Methods
The study included 41 eyes from 29 patients with DR who underwent FA and OCTA. Regions surrounded by arteries or veins were extracted in the OCTA image, and the FDs in each region were measured by Image J. Furthermore, each region was classified as NPA or perfused area (PA) in FA. The receiver operating characteristic (ROC) curve was prepared by logistic regression analysis of the FD. The AUC (area under the ROC curve) and cutoff value of FD were also calculated.
Results
Two hundred fifty-two regions were analyzed and classified into 38 NPA regions and 214 PA regions using FA. FD of each capillary plexus in NPA was significantly smaller than in PA (p < 0.0001). The AUC of total capillary plexus layers (TCP), superficial capillary plexus layer (SCP), and deep capillary plexus layer (DCP) was 0.975, 0.974, and 0.971, respectively. All areas, where the FD was more than the cutoff value (0.07 in TCP), were diagnosed with PA. Three areas with intraretinal microvascular abnormalities (IRMA) were diagnosed as PA despite being below the cutoff value.
Conclusions
FD measurement in OCTA is useful for NPA detection in DR.



Similar content being viewed by others
References
Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet 376:124–136. https://doi.org/10.1016/S0140-6736(09)62124-3
Bresnick GH, Palta M (1987) Predicting progression to severe proliferative diabetic retinopathy. Arch Ophthalmol 105:810–814
Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S (2012) Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol 96:694–698. https://doi.org/10.1136/bjophthalmol-2011-300774
Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS (2014) Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology 121:1783–1789. https://doi.org/10.1016/j.ophtha.2014.03.021
Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG (2013) Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology 120:795–802. https://doi.org/10.1016/j.ophtha.2012.09.032
Novotny HR, Alvis DL (1961) A method of photographing fluorescence in circulating blood in the human retina. Circulation 24:82–86
Early Treatment Diabetic Retinopathy Study Research Group (1991) Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Ophthalmology 98:807–822
Spaide RF, Klancnik JM Jr, Cooney MJ (2015) Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 133:45–50. https://doi.org/10.1001/jamaophthalmol.2014.3616
Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D (2012) Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 20:4710–4725. https://doi.org/10.1364/OE.20.004710
Agemy SA, Scripsema NK, Shah CM, Chui T, Garcia PM, Lee JG, Gentile RC, Hsiao YS, Zhou Q, Ko T, Rosen RB (2015) Retinal vascular perfusion density map** using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina 35:2353–2363. https://doi.org/10.1097/IAE.0000000000000862
Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y (2015) Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina 35:2377–2383. https://doi.org/10.1097/IAE.0000000000000849
Al-Sheikh M, Akil H, Pfau M, Sadda SR (2016) Swept-source OCT angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Invest Ophthalmol Vis Sci 57:3907–3913. https://doi.org/10.1167/iovs.16-19570
Miwa Y, Murakami T, Suzuma K, Uji A, Yoshitake S, Fujimoto M, Yoshitake T, Tamura Y, Yoshimura N (2016) Relationship between functional and structural changes in diabetic vessels in optical coherence tomography angiography. Sci Rep 6:29064. https://doi.org/10.1038/srep29064
Salz DA, de Carlo TE, Adhi M, Moult E, Choi W, Baumal CR, Witkin AJ, Duker JS, Fujimoto JG, Waheed NK (2016) Select features of diabetic retinopathy on swept-source optical coherence tomographic angiography compared with fluorescein angiography and normal eyes. JAMA Ophthalmol 134:644–650. https://doi.org/10.1001/jamaophthalmol.2016.0600
Munk MR, Giannakaki-Zimmermann H, Berger L, Huf W, Ebneter A, Wolf S, Zinkernagel MS (2017) OCT-angiography: a qualitative and quantitative comparison of 4 OCT-A devices. PLoS One 12:e0177059. https://doi.org/10.1371/journal.pone.0177059
Zweig MH, Campbell G (1993) Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39:561–577
Kaizu Y, Nakao S, Yoshida S, Hayami T, Arima M, Yamaguchi M, Wada I, Hisatomi T, Ikeda Y, Ishibashi T, Sonoda KH (2017) Optical coherence tomography angiography reveals spatial bias of macular capillary dropout in diabetic retinopathy. Invest Ophthalmol Vis Sci 58:4889–4897. https://doi.org/10.1167/iovs.17-22306
Niki T, Muraoka K, Shimizu K (1984) Distribution of capillary nonperfusion in early-stage diabetic retinopathy. Ophthalmology 91:1431–1439
Spaide RF, Fujimoto JG, Waheed NK (2015) Image artifacts in optical coherence tomography angiography. Retina 35:2163–2180. https://doi.org/10.1097/IAE.0000000000000765
Hwang TS, Gao SS, Liu L, Lauer AK, Bailey ST, Flaxel CJ, Wilson DJ, Huang D, Jia Y (2016) Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol 134:367–373. https://doi.org/10.1001/jamaophthalmol.2015.5658
Bresnick GH (1983) Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology 90:1301–1317
Writing Committee for the Diabetic Retinopathy Clinical Research N, Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL 3rd, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW (2015) Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 314:2137–2146. https://doi.org/10.1001/jama.2015.15217
Yamaguchi M, Nakao S, Arita R, Kaizu Y, Arima M, Zhou Y, Kita T, Yoshida S, Kimura K, Isobe T, Kaneko Y, Sonoda KH, Ishibashi T (2016) Vascular normalization by ROCK inhibitor: therapeutic potential of ripasudil (K-115) eye drop in retinal angiogenesis and hypoxia. Invest Ophthalmol Vis Sci 57:2264–2276. https://doi.org/10.1167/iovs.15-17411
Liu Y, Shen J, Fortmann SD, Wang J, Vestweber D, Campochiaro PA (2017) Reversible retinal vessel closure from VEGF-induced leukocyte plugging. JCI Insight 2. https://doi.org/10.1172/jci.insight.95530
Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP (2013) Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology 120:2587–2595. https://doi.org/10.1016/j.ophtha.2013.05.004
Acknowledgements
The authors thank Dr. Shigeo Yoshida for helpful suggestions.
Funding
This study was supported by grants from JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) No. 17K11456 (SN), Charitable Trust Fund for Ophthalmic Research in Commemoration of Santen Pharmaceutical’s Founder (SN), Novartis Pharma Research Grants (SN), and Alcon Pharma Research Grants (SN).
Author information
Authors and Affiliations
Contributions
YK and SN did the research of design, data acquisition and analysis, interpretation of data, drafting the manuscript, and critical revision of the manuscript. HS, IW, MY, TH, YI, and K-H S did the data acquisition and analysis, interpretation of data, and critical revision of the manuscript. JK did the analysis, interpretation of data, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Patient consent
Informed consent was obtained.
Ethics approval
This study was approved by the Ethics Committee of Kyushu University Hospital.
Additional information
Meeting Presentation: 50th Retina Society Annual Meeting
Electronic supplementary material
Supplemental Figure 1
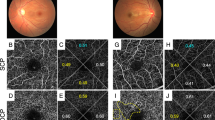
Flow density (FD) distribution of examined regions in all cases. Black circles and white triangles indicate perfused and nonperfused areas, respectively, which were judged using corresponding fluorescein angiography images. The vertical axis shows FD of total capillary plexus layers (TCP), and the horizontal axis shows all 29 cases included in this study. Dotted horizontal line indicates cutoff value of FD in TCP (0.07) with sensitivity (1.0) and specificity (0.95), respectively. (JPEG 597 kb)
Supplemental Figure 2
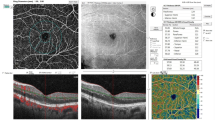
Decreased flow density (FD) in OCTA for unclear reasons, Case 4 (right eye). (A) Color fundus photograph shows proliferative diabetic retinopathy. (B) Fluorescein angiography (FA) shows new vessel growth on the disc and some microaneurysms. Yellow dot square indicates the magnified FA images in (C, D) and OCTA image in (E). (C, D) The area with blue dots was classified as perfused area in the early phase (C, 46 s) and the late phase (D, 7 min 55 s) of FA. (E) FD of area with blue dots in OCTA image is 0.002. (F) B scan of blue dots area. (JPEG 2441 kb)
Supplemental Figure 3
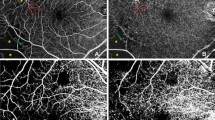
High hierarchical bias ratio of flow density (FD) between surface layer and deep layer, Case 1 (right eye). (A) Color fundus photograph shows proliferative diabetic retinopathy. (B) Fluorescein angiography (FA) shows some IRMA and microaneurysms. Yellow dot square indicates the magnified FA images in (C) and OCTA image in (D, E, F). (C) The area with blue dots was classified as perfused area in the early phase (C, 42 s) of FA. (D, E, F) FD of total capillary plexus (TCP, D), superficial capillary plexus (SCP, E) and deep capillary plexus (DCP, F) of area with blue dots in OCTA image is 0.155, 0.151 and 0.024, respectively. (F) B scan of blue dots area. (JPEG 2949 kb)
Rights and permissions
About this article
Cite this article
Kaizu, Y., Nakao, S., Sekiryu, H. et al. Retinal flow density by optical coherence tomography angiography is useful for detection of nonperfused areas in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 256, 2275–2282 (2018). https://doi.org/10.1007/s00417-018-4122-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4122-6