Abstract
Introduction
Loss of dorsolateral nigral hyperintensity (DNH) on iron-sensitive brain MRI is useful for Parkinson’s disease detection. DNH loss could also be of diagnostic value in dementia with Lewy bodies (DLB), an a-synuclein-related pathology. We aim to quantitatively synthesize evidence, investigating the role of MRI, a first-line imaging modality, in early DLB detection and differentiation from other dementias.
Methods
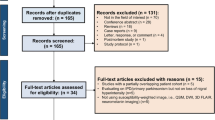
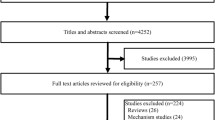
Our study was conducted according to the PRISMA statement. MEDLINE, Scopus, Web of Science, and Cochrane Library were searched using the terms like “dementia with Lewy bodies”, “dorsolateral nigral hyperintensity”, and “MRI”. Only English-written peer-reviewed diagnostic accuracy studies were included. We used QUADAS-2 for quality assessment.
Results
Our search yielded 363 search results. Three studies were eligible, all with satisfying, high quality. The total population of 227 patients included 63 with DLB and 164 with other diseases (Alzheimer disease, frontotemporal dementia, mild cognitive impairment). Using a univariate random-effects logistic regression model, our meta-analysis resulted in pooled sensitivity, specificity and DOR of 0.82 [0.62; 0.92], 0.79 [0.70; 0.86] and 16.26 ([3.3276; 79.4702], p = 0.0006), respectively, for scans with mixed field strength (1.5 and 3 T). Subgroup analysis of 3 T scans showed pooled sensitivity, specificity and DOR of 0.82 [0.61; 0.93], 0.82 [0.72; 0.89] and 18.36 ([4.24; 79.46], p < 0.0001), respectively.
Discussion
DNH loss on iron-sensitive MRI might comprise a supportive biomarker for DLB detection, that could augment the value of the DLB diagnostic criteria. Further evaluation using standardized protocols is needed, as well as direct comparison to other supportive and indicative biomarkers.






Similar content being viewed by others
Data availability
Not applicable.
References
McKeith IG et al (2017) Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89(1):88. https://doi.org/10.1212/WNL.0000000000004058
Haller S et al (2013) Neuroimaging of dementia in 2013: what radiologists need to know. Eur Radiol 23(12):3393–3404. https://doi.org/10.1007/S00330-013-2957-0
McKeith I (2004) Dementia with Lewy bodies. Dialogues Clin Neurosci 6(3):333–341. https://doi.org/10.31887/DCNS.2004.6.3/IMCKEITH
Outeiro TF et al (2019) Dementia with Lewy bodies: an update and outlook. Mol Neurodegener. https://doi.org/10.1186/S13024-019-0306-8
Blazejewska AI et al (2013) Visualization of nigrosome 1 and its loss in PD: pathoanatomical correlation and in vivo 7 T MRI. Neurology 81(6):534–540. https://doi.org/10.1212/WNL.0B013E31829E6FD2
Chau MT, Todd G, Wilcox R, Agzarian M, Bezak E (2020) Diagnostic accuracy of the appearance of Nigrosome-1 on magnetic resonance imaging in Parkinson’s disease: a systematic review and meta-analysis. Parkinson Relat Disord 78:12–20. https://doi.org/10.1016/J.PARKRELDIS.2020.07.002
** L et al (2019) Combined visualization of nigrosome-1 and neuromelanin in the substantia Nigra using 3T MRI for the differential diagnosis of essential tremor and de novo Parkinson’s disease. Front Neurol 10(Feb):100. https://doi.org/10.3389/FNEUR.2019.00100
Kim EY, Sung YH, Shin HG, Noh Y, Nam Y, Lee J (2018) Diagnosis of early-stage idiopathic Parkinson’s disease using high-resolution quantitative susceptibility map** combined with histogram analysis in the substantia Nigra at 3 T. J Clin Neurol 14(1):90. https://doi.org/10.3988/JCN.2018.14.1.90
Stezin A et al (2018) Clinical utility of visualisation of nigrosome-1 in patients with Parkinson’s disease. Eur Radiol 28(2):718–726. https://doi.org/10.1007/S00330-017-4950-5
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122(Pt 8):1437–1448. https://doi.org/10.1093/BRAIN/122.8.1437
Gao P et al (2015) Visualization of nigrosomes-1 in 3T MR susceptibility weighted imaging and its absence in diagnosing Parkinson’s disease. Eur Rev Med Pharmacol Sci 19(23):4603–4609
Kim JM et al (2016) Loss of substantia nigra hyperintensity on 7 Tesla MRI of Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Parkinson Relat Disord 26:47–54. https://doi.org/10.1016/J.PARKRELDIS.2016.01.023
Bae YJ et al (2016) Loss of nigral hyperintensity on 3 tesla MRI of Parkinsonism: comparison with (123) I-FP-CIT SPECT. Mov Disord 31(5):684–692. https://doi.org/10.1002/MDS.26584
Kwon DH et al (2012) Seven-Tesla magnetic resonance images of the substantia Nigra in Parkinson disease. Ann Neurol 71(2):267–277. https://doi.org/10.1002/ANA.22592
Noh Y, Sung YH, Lee J, Kim EY (2015) Nigrosome 1 detection at 3T MRI for the diagnosis of early-stage idiopathic Parkinson disease: assessment of diagnostic accuracy and agreement on imaging asymmetry and clinical laterality. AJNR Am J Neuroradiol 36(11):2010–2016. https://doi.org/10.3174/AJNR.A4412
Pyatigorskaya N et al (2018) Comparative study of MRI biomarkers in the substantia Nigra to discriminate idiopathic Parkinson disease. AJNR Am J Neuroradiol 39(8):1460–1467. https://doi.org/10.3174/AJNR.A5702
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. https://doi.org/10.1371/JOURNAL.PMED.1000097
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. https://doi.org/10.1136/BMJ.N71
Clark J, Glasziou P, Del Mar C, Bannach-Brown A, Stehlik P, Scott AM (2020) A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol 121:81–90. https://doi.org/10.1016/J.JCLINEPI.2020.01.008
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5(1):1–10. https://doi.org/10.1186/S13643-016-0384-4/FIGURES/6
Haddaway NR, Page MJ, Pritchard CC, McGuinness LA (2022) PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev 18(2):e1230. https://doi.org/10.1002/CL2.1230
Whiting PF et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Takwoingi Y, Guo B, Riley RD, Deeks JJ (2017) Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Stat Methods Med Res 26(4):1896–1911. https://doi.org/10.1177/0962280215592269
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82(13):1–26. https://doi.org/10.18637/JSS.V082.I13
Kamagata K et al (2017) Diagnostic imaging of dementia with Lewy bodies by susceptibility-weighted imaging of nigrosomes versus striatal dopamine transporter single-photon emission computed tomography: a retrospective observational study. Neuroradiology 59(1):89–98. https://doi.org/10.1007/S00234-016-1773-Z
Shams S et al (2017) MRI of the swallow tail sign: A useful marker in the diagnosis of Lewy body dementia? AJNR Am J Neuroradiol 38(9):1737–1741. https://doi.org/10.3174/AJNR.A5274
Rizzo G et al (2019) Loss of swallow tail sign on susceptibility-weighted imaging in dementia with Lewy bodies. J Alzheimers Dis 67(1):61–65. https://doi.org/10.3233/JAD-180687
McKeith IG et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65(12):1863–1872. https://doi.org/10.1212/01.WNL.0000187889.17253.B1
Cronin P, Kelly AM, Altaee D, Foerster B, Petrou M, Dwamena BA (2018) How to perform a systematic review and meta-analysis of diagnostic imaging studies. Acad Radiol 25(5):573–593. https://doi.org/10.1016/J.ACRA.2017.12.007
Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58(10):982–990. https://doi.org/10.1016/J.JCLINEPI.2005.02.022
De Pietro Franco Zorzenon C, Almeida Antônio Bienes GH, Duarte Alves E, Tobaru Tibana LA, Carrete Júnior H, Ballalai Ferraz H (2021) Magnetic resonance imaging evaluation of nigrosome 1 and neuromelanin can assist Parkinson’s disease diagnosis, but requires an expert neuroradiologist. Parkinson Relat Disord 83:8–12. https://doi.org/10.1016/J.PARKRELDIS.2020.12.006
Cheng Z et al (2020) Imaging the Nigrosome 1 in the substantia Nigra using susceptibility weighted imaging and quantitative susceptibility map**: an application to Parkinson’s disease. Neuroimage Clin. https://doi.org/10.1016/J.NICL.2019.102103
Burton EJ et al (2009) Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 132(Pt 1):195–203. https://doi.org/10.1093/BRAIN/AWN298
Harper L et al (2016) MRI visual rating scales in the diagnosis of dementia: evaluation in 184 post-mortem confirmed cases. Brain 139(Pt 4):1211–1225. https://doi.org/10.1093/BRAIN/AWW005
Nedelska Z et al (2015) Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol Aging 36(1):452. https://doi.org/10.1016/J.NEUROBIOLAGING.2014.07.005
Lobotesis K et al (2001) Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 56(5):643–649. https://doi.org/10.1212/WNL.56.5.643
O’Brien JT et al (2014) 18F-FDG PET and perfusion SPECT in the diagnosis of Alzheimer and Lewy body dementias. J Nucl Med 55(12):1959–1965. https://doi.org/10.2967/JNUMED.114.143347
De Marzi R et al (2016) Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 79(6):1026–1030. https://doi.org/10.1002/ANA.24646
Bae YJ et al (2018) Loss of substantia nigra hyperintensity at 3.0-T MR imaging in idiopathic REM sleep behavior disorder: comparison with 123I-FP-CIT SPECT. Radiology 287(1):285–293. https://doi.org/10.1148/RADIOL.2017162486
Funding
The publication of the article in OA mode was financially supported by HEAL-Link.
Author information
Authors and Affiliations
Contributions
Conceptualization: VST, KE; Literature review: VST, GK, DDC, TM; Data analysis: VST, GK; Writing—original draft preparation: VST, KE, PP, CP; Writing—review and editing: VST, TM, MA, SK; Supervision: SK.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tseriotis, VS., Mavridis, T., Eleftheriadou, K. et al. Loss of the “swallow tail sign” on susceptibility-weighted imaging in the diagnosis of dementia with Lewy bodies: a systematic review and meta-analysis. J Neurol (2024). https://doi.org/10.1007/s00415-024-12381-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12381-6