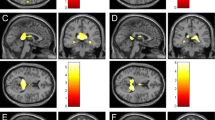
Abstract
Background
Frontal cortico-subcortical dysfunction may contribute to fatigue and dual-task impairment of walking and cognition in progressive multiple sclerosis (PMS).
Purpose
To explore the associations among fatigue, dual-task performance and structural and functional abnormalities of frontal cortico-subcortical network in PMS.
Methods
Brain 3 T structural and functional MRI sequences, Modified Fatigue Impact Scale (MFIS), dual-task motor and cognitive performances were obtained from 57 PMS patients and 10 healthy controls (HC). The associations of thalamic, caudate nucleus and dorsolateral prefrontal cortex (DLPFC) atrophy, microstructural abnormalities of their connections and their resting state effective connectivity (RS-EC) with fatigue and dual-task performance were investigated using random forest.
Results
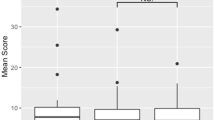
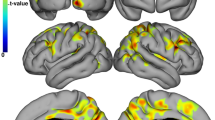
Thirty-seven PMS patients were fatigued (F) (MFIS ≥ 38). Compared to HC, non-fatigued (nF) and F-PMS patients had significantly worse dual-task performance (p ≤ 0.002). Predictors of fatigue (out-of-bag [OOB]-accuracy = 0.754) and its severity (OOB-R2 = 0.247) were higher Expanded Disability Status scale (EDSS) score, lower RS-EC from left-caudate nucleus to left-DLPFC, lower fractional anisotropy between left-caudate nucleus and left-thalamus, higher mean diffusivity between right-caudate nucleus and right-thalamus, and longer disease duration. Microstructural abnormalities in connections among thalami, caudate nuclei and DLPFC, mainly left-lateralized in nF-PMS and more bilateral in F-PMS, higher RS-EC from left-DLPFC to right-DLPFC in nF-PMS and lower RS-EC from left-caudate nucleus to left-DLPFC in F-PMS, higher EDSS score, higher WM lesion volume, and lower cortical volume predicted worse dual-task performances (OOB-R2 from 0.426 to 0.530).
Conclusions
In PMS, structural and functional frontal cortico-subcortical abnormalities contribute to fatigue and worse dual-task performance, with different patterns according to the presence of fatigue.



Similar content being viewed by others
Data availability
The dataset used and analyzed during the current study is available from the corresponding author on reasonable request.
References
Marchesi O, Vizzino C, Filippi M, Rocca MA (2022) Current perspectives on the diagnosis and management of fatigue in multiple sclerosis. Expert Rev Neurother 22(8):681–693
Arm J, Ribbons K, Lechner-Scott J, Ramadan S (2019) Evaluation of MS related central fatigue using MR neuroimaging methods: sco** review. J Neurol Sci 400:52–71
Bertoli M, Tecchio F (2020) Fatigue in multiple sclerosis: does the functional or structural damage prevail? Mult Scler 26(14):1809–1815
Filippi M, Preziosa P, Rocca MA (2019) Brain map** in multiple sclerosis: lessons learned about the human brain. Neuroimage 190:32–45
Leone C, Feys P, Moumdjian L, D’Amico E, Zappia M, Patti F (2017) Cognitive-motor dual-task interference: a systematic review of neural correlates. Neurosci Biobehav Rev 75:348–360
Postigo-Alonso B, Galvao-Carmona A, Benitez I, Conde-Gavilan C, Jover A, Molina S et al (2018) Cognitive-motor interference during gait in patients with multiple sclerosis: a mixed methods systematic review. Neurosci Biobehav Rev 94:126–148
Wajda DA, Sosnoff JJ (2015) Cognitive-motor interference in multiple sclerosis: a systematic review of evidence, correlates, and consequences. Biomed Res Int 2015:720856
Learmonth YC, Ensari I, Motl RW (2017) Cognitive motor interference in multiple sclerosis: insights from a systematic quantitative review. Arch Phys Med Rehabil 98(6):1229–1240
Veldkamp R, Goetschalckx M, Hulst HE, Nieuwboer A, Grieten K, Baert I et al (2021) Cognitive-motor interference in individuals with a neurologic disorder: a systematic review of neural correlates. Cogn Behav Neurol 34(2):79–95
Hernandez ME, Holtzer R, Chaparro G, Jean K, Balto JM, Sandroff BM et al (2016) Brain activation changes during locomotion in middle-aged to older adults with multiple sclerosis. J Neurol Sci 370:277–283
Chaparro G, Balto JM, Sandroff BM, Holtzer R, Izzetoglu M, Motl RW et al (2017) Frontal brain activation changes due to dual-tasking under partial body weight support conditions in older adults with multiple sclerosis. J Neuroeng Rehabil 14(1):65
Saleh S, Sandroff BM, Vitiello T, Owoeye O, Hoxha A, Hake P et al (2018) The Role of premotor areas in dual tasking in healthy controls and persons with multiple sclerosis: an fNIRS imaging study. Front Behav Neurosci 12:296
Wolkorte R, Heersema DJ, Zijdewind I (2015) Reduced dual-task performance in MS patients is further decreased by muscle fatigue. Neurorehabil Neural Repair 29(5):424–435
Feinstein A, Amato MP, Brichetto G, Chataway J, Chiaravalloti N, Dalgas U et al (2020) Study protocol: improving cognition in people with progressive multiple sclerosis: a multi-arm, randomized, blinded, sham-controlled trial of cognitive rehabilitation and aerobic exercise (COGEx). BMC Neurol 20(1):204
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ et al (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83(3):278–286
Sacco R, Santangelo G, Stamenova S, Bisecco A, Bonavita S, Lavorgna L et al (2016) Psychometric properties and validity of Beck Depression Inventory II in multiple sclerosis. Eur J Neurol 23(4):744–750
Strober L, DeLuca J, Benedict RH, Jacobs A, Cohen JA, Chiaravalloti N et al (2019) Symbol Digit Modalities Test: a valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult Scler 25(13):1781–1790
Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF (1994) Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis 18(Suppl 1):S79-83
Flachenecker P, Kumpfel T, Kallmann B, Gottschalk M, Grauer O, Rieckmann P et al (2002) Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler 8(6):523–526
Learmonth YC, Sandroff BM, Pilutti LA, Klaren RE, Ensari I, Riskin BJ et al (2014) Cognitive motor interference during walking in multiple sclerosis using an alternate-letter alphabet task. Arch Phys Med Rehabil 95(8):1498–1503
Valverde S, Cabezas M, Roura E, Gonzalez-Villa S, Pareto D, Vilanova JC et al (2017) Improving automated multiple sclerosis lesion segmentation with a cascaded 3D convolutional neural network approach. Neuroimage 155:159–168
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219
Zhang H, Avants BB, Yushkevich PA, Woo JH, Wang S, McCluskey LF et al (2007) High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: an example study using amyotrophic lateral sclerosis. IEEE Trans Med Imaging 26(11):1585–1597
Zhang H, Yushkevich PA, Rueckert D, Gee JC (2007) Unbiased white matter atlas construction using diffusion tensor images. Med Image Comput Comput Assist Interv 10(Pt 2):211–218
Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J (2014) Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 103:411–426
Pravata E, Zecca C, Sestieri C, Caulo M, Riccitelli GC, Rocca MA et al (2016) Hyperconnectivity of the dorsolateral prefrontal cortex following mental effort in multiple sclerosis patients with cognitive fatigue. Mult Scler 22(13):1665–1675
Jaeger S, Paul F, Scheel M, Brandt A, Heine J, Pach D et al (2019) Multiple sclerosis-related fatigue: Altered resting-state functional connectivity of the ventral striatum and dorsolateral prefrontal cortex. Mult Scler 25(4):554–564
Friston KJ, Harrison L, Penny W (2003) Dynamic causal modelling. Neuroimage 19(4):1273–1302
Friston KJ, Kahan J, Biswal B, Razi A (2014) A DCM for resting state fMRI. Neuroimage 94:396–407
Zeidman P, Jafarian A, Corbin N, Seghier ML, Razi A, Price CJ et al (2019) A guide to group effective connectivity analysis, part 1: first level analysis with DCM for fMRI. Neuroimage 200:174–190
Kursa MB, Rudnicki WR (2010) Feature selection with the Boruta Package. J Stat Softw 36(11):1–13
Marchesi O, Vizzino C, Meani A, Conti L, Riccitelli GC, Preziosa P et al (2020) Fatigue in multiple sclerosis patients with different clinical phenotypes: a clinical and magnetic resonance imaging study. Eur J Neurol 27(12):2549–2560
Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G et al (2002) Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage 15(3):559–567
DeLuca J, Genova HM, Hillary FG, Wylie G (2008) Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. J Neurol Sci 270(1–2):28–39
Rocca MA, Gatti R, Agosta F, Broglia P, Rossi P, Riboldi E et al (2009) Influence of task complexity during coordinated hand and foot movements in MS patients with and without fatigue. A kinematic and functional MRI study. J Neurol 256(3):470–482
Engstrom M, Flensner G, Landtblom AM, Ek AC, Karlsson T (2013) Thalamo-striato-cortical determinants to fatigue in multiple sclerosis. Brain Behav 3(6):715–728
Damasceno A, Damasceno BP, Cendes F (2016) Atrophy of reward-related striatal structures in fatigued MS patients is independent of physical disability. Mult Scler 22(6):822–829
Hidalgo de la Cruz M, d’Ambrosio A, Valsasina P, Pagani E, Colombo B, Rodegher M et al (2018) Abnormal functional connectivity of thalamic sub-regions contributes to fatigue in multiple sclerosis. Mult Scler 24(9):1183–1195
Genova HM, Rajagopalan V, Deluca J, Das A, Binder A, Arjunan A et al (2013) Examination of cognitive fatigue in multiple sclerosis using functional magnetic resonance imaging and diffusion tensor imaging. PLoS ONE 8(11):e78811
Finke C, Schlichting J, Papazoglou S, Scheel M, Freing A, Soemmer C et al (2015) Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult Scler 21(7):925–934
Pardini M, Bonzano L, Bergamino M, Bommarito G, Feraco P, Murugavel A et al (2015) Cingulum bundle alterations underlie subjective fatigue in multiple sclerosis. Mult Scler 21(4):442–447
Roelcke U, Kappos L, Lechner-Scott J, Brunnschweiler H, Huber S, Ammann W et al (1997) Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology 48(6):1566–1571
Rocca MA, Meani A, Riccitelli GC, Colombo B, Rodegher M, Falini A et al (2016) Abnormal adaptation over time of motor network recruitment in multiple sclerosis patients with fatigue. Mult Scler 22(9):1144–1153
Stephan KE, Friston KJ (2010) Analyzing effective connectivity with functional magnetic resonance imaging. Wiley Interdiscip Rev Cogn Sci 1(3):446–459
Rocca MA, Valsasina P, Colombo B, Martinelli V, Filippi M (2021) Cortico-subcortical functional connectivity modifications in fatigued multiple sclerosis patients treated with fampridine and amantadine. Eur J Neurol 28(7):2249–2258
Ruggieri S, Fanelli F, Castelli L, Petsas N, De Giglio L, Prosperini L (2018) Lesion symptom map of cognitive-postural interference in multiple sclerosis. Mult Scler 24(5):653–662
Tartaglia MC, Narayanan S, Arnold DL (2008) Mental fatigue alters the pattern and increases the volume of cerebral activation required for a motor task in multiple sclerosis patients with fatigue. Eur J Neurol 15(4):413–419
Chen MH, DeLuca J, Genova HM, Yao B, Wylie GR (2020) Cognitive fatigue is associated with altered functional connectivity in interoceptive and reward pathways in multiple sclerosis. Diagnostics (Basel). 10(11):930
Chen MH, Wylie GR, Sandroff BM, Dacosta-Aguayo R, DeLuca J, Genova HM (2020) Neural mechanisms underlying state mental fatigue in multiple sclerosis: a pilot study. J Neurol 267(8):2372–2382
Au Duong MV, Boulanouar K, Audoin B, Treseras S, Ibarrola D, Malikova I et al (2005) Modulation of effective connectivity inside the working memory network in patients at the earliest stage of multiple sclerosis. Neuroimage 24(2):533–538
Dobryakova E, Rocca MA, Valsasina P, Ghezzi A, Colombo B, Martinelli V et al (2016) Abnormalities of the executive control network in multiple sclerosis phenotypes: an fMRI effective connectivity study. Hum Brain Mapp 37(6):2293–2304
Dobryakova E, Rocca MA, Valsasina P, DeLuca J, Filippi M (2017) Altered neural mechanisms of cognitive control in patients with primary progressive multiple sclerosis: an effective connectivity study. Hum Brain Mapp 38(5):2580–2588
Meng D, Welton T, Elsarraj A, Morgan PS, das Nair R, Constantinescu CS et al (2021) Dorsolateral prefrontal circuit effective connectivity mediates the relationship between white matter structure and PASAT-3 performance in multiple sclerosis. Hum Brain Mapp 42(2):495–509
Cordani C, Preziosa P, Valsasina P, Meani A, Pagani E, Morozumi T et al (2022) MRI of transcallosal white matter helps to predict motor impairment in multiple sclerosis. Radiology 302(3):639–649
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was funded by a grant from the Multiple Sclerosis Society of Canada (Grant No. #EGID3185) and the National MS Society.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Paolo Preziosa received speaker honoraria from Roche, Biogen, Novartis, Merck Serono, Bristol Myers Squibb and Genzyme. He has received research support from Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. Maria Assunta Rocca received speaker honoraria from Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, Roche, and Teva, and receives research support from the MS Society of Canada and Fondazione Italiana Sclerosi Multipla. Elisabetta Pagani, Paola Valsasina, Nicolò Bruschi, Alessandro Meani, Cecilia Meza, Robert W. Motl and Brian Sandroff have nothing to disclose. Maria Pia Amato received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Roche, Pharmaceutical Industries and Fondazione Italiana Sclerosi Multipla. Giampaolo Brichetto has been awarded and receives research support from Roche, Fondazione Italiana Sclerosi Multipla, ARSEP, H2020 EU Call. Jeremy Chataway has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership and the Health Technology Assessment (HTA) Programme (NIHR), the UK MS Society, the US National MS Society and the Rosetrees Trust. He is supported in part by the NIHR University College London Hospitals (UCLH) Biomedical Research Centre, London, UK. He has been a local principal investigator for a trial in MS funded by the Canadian MS society. A local principal investigator for commercial trials funded by: Actelion, Novartis and Roche; and has taken part in advisory boards/consultancy for Azadyne, Janssen, Merck, NervGen, Novartis and Roche. Nancy D. Chiaravalloti is on an Advisory Board for Akili Interactive and is a member of the Editorial Boards of Multiple Sclerosis Journal and Frontiers in NeuroTrauma. Gary Cutter is a member of Data and Safety Monitoring Boards for Astra-Zeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Horizon Pharmaceuticals,Hisun Pharmaceuticals, Mapi Pharmaceuticals LTD, Merck, Merck/Pfizer, Opko Biologics, OncoImmune, Neurim, Novartis, Ophazyme, Sanofi Aventis, Reata Pharmaceuticals, Teva pharmaceuticals, VielaBio Inc, Vivus, NHLBI (Protocol Review Committee), NICHD (OPRU oversight committee). He is on Consulting or Advisory Boards for Biodelivery Sciences International, Biogen, Click Therapeutics, Genzyme, Genentech, GW Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Medday, Neurogenesis LTD, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion/Cerexis Pharmaceuticals, Roche, TG Therapeutics. Dr. Cutter is employed by the University of Alabama at Birmingham and President of Pythagoras, Inc. a private consulting company located in Birmingham AL. Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck-Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme. John DeLuca is an Associate Editor of the Archives of Physical Medicine and Rehabilitation, and Neuropsychology Review; received compensation for consulting services and/or speaking activities from Biogen Idec, Celgene, MedRhythms, and Novartis; and receives research support from Biogen Idec, National Multiple Sclerosis Society, Consortium of Multiple Sclerosis Centers, and National Institutes of Health. Rachel Farrell has received honoraria and served on advisory panels for Merck, TEVA, Novartis, Genzyme, GW pharma (Jazz pharmaceuticals), Allergan, Merz, Ipsen and Biogen. She is supported in part by the National Institute for Health Research, University College London Hospitals, Biomedical Research Centre, London, UK. Peter Feys is editorial board member of NNR and MSJ, provides consultancy to NeuroCompass and was board of advisory board meetings for BIOGEN. Jennifer Freeman has been awarded research grants from the NIHR, UK. Matilde Inglese is Co-Editor for Controversies for Multiple Sclerosis Journal; received compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme; and received research support from NIH, NMSS, the MS Society of Canada, the Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, H2020 EU Call. Amber Salter receives research funding from Multiple Sclerosis Society of Canada, National Multiple Sclerosis Society, CMSC and the US Department of Defense and is a member of editorial board for Neurology. Anthony Feinstein is on Advisory Boards for Akili Interactive and Roche, and reports grants from the MS Society of Canada, book royalties from Johns Hopkins University Press, Cambridge University Press, Amadeus Press and Glitterati Editions, and speaker’s honoraria from Novartis, Biogen, Roche and Sanofi Genzyme. Massimo Filippi is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Map**, Associate Editor of Radiology, and Associate Editor of Neurological Sciences; received compensation for consulting services and/or speaking activities from Alexion, Almirall, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA).
Ethical approval
Approval was received from the local institutional ethical standards committees on human experimentation for any experiments using human subjects. Written informed consent was obtained from all subjects prior to study participation according to the Declaration of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Preziosa, P., Rocca, M.A., Pagani, E. et al. Structural and functional magnetic resonance imaging correlates of fatigue and dual-task performance in progressive multiple sclerosis. J Neurol 270, 1543–1563 (2023). https://doi.org/10.1007/s00415-022-11486-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11486-0