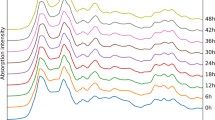
Abstract
Objective
The aim of this study is to identify a rapid, sensitive, and non-destructive auxiliary approach for postmortem diagnosis of SCD, addressing the challenges faced in forensic practice.
Methods
ATR-FTIR spectroscopy was employed to collect spectral features of blood samples from different cases, combined with pathological changes. Mixed datasets were analyzed using ANN, KNN, RF, and SVM algorithms. Evaluation metrics such as accuracy, precision, recall, F1-score and confusion matrix were used to select the optimal algorithm and construct the postmortem diagnosis model for SCD.
Results
A total of 77 cases were collected, including 43 cases in the SCD group and 34 cases in the non-SCD group. A total of 693 spectrogram were obtained. Compared to other algorithms, the SVM algorithm demonstrated the highest accuracy, reaching 95.83% based on spectral biomarkers. Furthermore, by combing spectral biomarkers with age, gender, and cardiac histopathological changes, the accuracy of the SVM model could get 100%.
Conclusion
Integrating artificial intelligence technology, pathology, and physical chemistry analysis of blood components can serve as an effective auxiliary method for postmortem diagnosis of SCD.




Similar content being viewed by others
Data availability
The data supporting the conclusions are included in the article. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Zipes DP et al (2006) ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 48(5):e247-346. https://doi.org/10.1016/j.jacc.2006.07.010
Kandala J, Oommen C, Kern KB (2017) Sudden cardiac death. Br Med Bull 122(1):5–15. https://doi.org/10.1093/bmb/ldx011
Wellens HJ et al (2014) Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 35(25):1642–1651. https://doi.org/10.1093/eurheartj/ehu176
Monserrat L et al (2003) Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol 42(5):873–879. https://doi.org/10.1016/s0735-1097(03)00827-1
Corrado D, Link MS, Calkins H (2017) Arrhythmogenic Right Ventricular Cardiomyopathy. N Engl J Med 376(1):61–72. https://doi.org/10.1056/nejmra1509267
Myerburg RJ (2001) Sudden cardiac death: exploring the limits of our knowledge. J Cardiovasc Electrophysiol 12(3):369–381. https://doi.org/10.1046/j.1540-8167.2001.00369.x
Stecker EC et al (2006) Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 47(6):1161–1166. https://doi.org/10.1016/j.jacc.2005.11.045
Khairy P et al (2022) Sudden cardiac death in congenital heart disease. Eur Heart J 43(22):2103–2115. https://doi.org/10.1093/eurheartj/ehac104
Basso C et al (2010) Guidelines for autopsy investigation of sudden cardiac death. Pathologica 102(5):391–404
Cao, Z., et al. (2019) Diagnostic Roles of Postmortem cTn I and cTn T in Cardiac Death with Special Regard to Myocardial Infarction: A Systematic Literature Review and Meta-Analysis. Int J Mol Sci 20(13). https://doi.org/10.3390/ijms20133351
Esmaeilzadeh M et al (2022) A Combined Echocardiography Approach for the Diagnosis of Cancer Therapy-Related Cardiac Dysfunction in Women With Early-Stage Breast Cancer. JAMA Cardiol 7(3):330–340. https://doi.org/10.1001/jamacardio.2021.5881
Carvajal-Zarrabal O et al (2017) Use of Cardiac Injury Markers in the Postmortem Diagnosis of Sudden Cardiac Death. J Forensic Sci 62(5):1332–1335. https://doi.org/10.1111/1556-4029.13397
Osman J et al (2019) Sudden Cardiac Death (SCD) - risk stratification and prediction with molecular biomarkers. J Biomed Sci 26(1):39. https://doi.org/10.1186/s12929-019-0535-8
Jouven X et al (2001) Circulating nonesterified fatty acid level as a predictive risk factor for sudden death in the population. Circulation 104(7):756–761. https://doi.org/10.1161/hc3201.094151
Tian, M, Cao Z, Pang H (2021) Circular RNAs in Sudden Cardiac Death Related Diseases: Novel Biomarker for Clinical and Forensic Diagnosis. Molecules, 26(4). https://doi.org/10.3390/molecules26041155
Sabatasso S et al (2016) Early markers for myocardial ischemia and sudden cardiac death. Int J Legal Med 130(5):1265–1280. https://doi.org/10.1007/s00414-016-1401-9
Polacco M et al (2015) Visualization of myocardial infarction by post-mortem single-organ coronary computed tomography: a feasibility study. Int J Legal Med 129(3):517–524. https://doi.org/10.1007/s00414-014-1085-y
Arrive L et al (2016) Postmortem coronary CT angiography. Intensive Care Med 42(8):1293–1294. https://doi.org/10.1007/s00134-016-4376-6
de la Grandmaison GL (2006) Is there progress in the autopsy diagnosis of sudden unexpected death in adults? Forensic Sci Int 156(2–3):138–144. https://doi.org/10.1016/j.forsciint.2004.12.024
Duckworth E et al (2022) Improving Vibrational Spectroscopy Prospects in Frontline Clinical Diagnosis: Fourier Transform Infrared on Buccal Mucosa Cancer. Anal Chem 94(40):13642–13646. https://doi.org/10.1021/acs.analchem.2c02496
Wang, R, Wang Y (2021) Fourier Transform Infrared Spectroscopy in Oral Cancer Diagnosis. Int J Mol Sci, 22(3). https://doi.org/10.3390/ijms22031206
Roy S et al (2017) Simultaneous ATR-FTIR Based Determination of Malaria Parasitemia, Glucose and Urea in Whole Blood Dried onto a Glass Slide. Anal Chem 89(10):5238–5245. https://doi.org/10.1021/acs.analchem.6b04578
Guang P et al (2020) Blood-based FTIR-ATR spectroscopy coupled with extreme gradient boosting for the diagnosis of type 2 diabetes: A STARD compliant diagnosis research. Medicine (Baltimore) 99(15):e19657. https://doi.org/10.1097/md.0000000000019657
Mateus PDSN et al (2023) Detection of metabolic syndrome with ATR-FTIR spectroscopy and chemometrics in blood plasma. Spectrochim Acta A Mol Biomol Spectrosc 288:122135. https://doi.org/10.1016/j.saa.2022.122135
Lin H et al (2018) Identification of Pulmonary Edema in Forensic Autopsy Cases of Sudden Cardiac Death Using Fourier Transform Infrared Microspectroscopy: A Pilot Study. Anal Chem 90(4):2708–2715. https://doi.org/10.1021/acs.analchem.7b04642
Dorling KM, Baker MJ (2013) Highlighting attenuated total reflection Fourier transform infrared spectroscopy for rapid serum analysis. Trends Biotechnol 31(6):327–328. https://doi.org/10.1016/j.tibtech.2013.03.010
Morais C et al (2020) Tutorial: multivariate classification for vibrational spectroscopy in biological samples. Nat Protoc 15(7):2143–2162. https://doi.org/10.1038/s41596-020-0322-8
Villamanca JJ et al (2022) Predicting the Likelihood of Colorectal Cancer with Artificial Intelligence Tools Using Fourier Transform Infrared Signals Obtained from Tumor Samples. Appl Spectrosc 76(12):1412–1428. https://doi.org/10.1177/00037028221116083
Ho CS et al (2019) Rapid identification of pathogenic bacteria using Raman spectroscopy and deep learning. Nat Commun 10(1):4927. https://doi.org/10.1038/s41467-019-12898-9
Jadhav SA et al (2021) Development of integrated microfluidic platform coupled with Surface-enhanced Raman Spectroscopy for diagnosis of COVID-19. Med Hypotheses 146:110356. https://doi.org/10.1016/j.mehy.2020.110356
Greener JG et al (2022) A guide to machine learning for biologists. Nat Rev Mol Cell Biol 23(1):40–55. https://doi.org/10.1038/s41580-021-00407-0
Ringner M (2008) What is principal component analysis? Nat Biotechnol 26(3):303–304. https://doi.org/10.1038/nbt0308-303
Yang Q et al (2017) Detection of inborn errors of metabolism utilizing GC-MS urinary metabolomics coupled with a modified orthogonal partial least squares discriminant analysis. Talanta 165:545–552. https://doi.org/10.1016/j.talanta.2017.01.018
Yang X et al (2022) Identification of myocardial fibrosis by ATR-FTIR spectroscopy combined with chemometrics. Spectrochim Acta A Mol Biomol Spectrosc 264:120238. https://doi.org/10.1016/j.saa.2021.120238
Tombolesi N et al (2022) Early cardiac-chamber-specific fingerprints in heart failure with preserved ejection fraction detected by FTIR and Raman spectroscopic techniques. Sci Rep 12(1):3440. https://doi.org/10.1038/s41598-022-07390-2
Paraskevaidi M et al (2017) Differential diagnosis of Alzheimer’s disease using spectrochemical analysis of blood. Proc Natl Acad Sci U S A 114(38):E7929–E7938. https://doi.org/10.1073/pnas.1701517114
Guo, S, et al. (2022) Fast and Deep Diagnosis Using Blood-Based ATR-FTIR Spectroscopy for Digestive Tract Cancers. Biomolecules, 12(12). https://doi.org/10.3390/biom12121815
Davies MJ et al (1989) Factors influencing the presence or absence of acute coronary artery thrombi in sudden ischaemic death. Eur Heart J 10(3):203–208. https://doi.org/10.1093/oxfordjournals.eurheartj.a059467
Holmstrom L et al (2022) Plaque histology and myocardial disease in sudden coronary death: the Fingesture study. Eur Heart J 43(47):4923–4930. https://doi.org/10.1093/eurheartj/ehac533
Holmstrom L et al (2020) Electrocardiographic associations with myocardial fibrosis among sudden cardiac death victims. Heart 106(13):1001–1006. https://doi.org/10.1136/heartjnl-2019-316105
Nedaie A, Najafi AA (2018) Support vector machine with Dirichlet feature map**. Neural Netw 98:87–101. https://doi.org/10.1016/j.neunet.2017.11.006
**g-yi TAN CCLA (2023) Case Study of Coronary Heart Disease Classification Prediction Based on SVM Alg. J Med Inf 36(01):37–41
Acknowledgements
The authors thank the patients and their families for participating in this study.
Funding
This research was supported by grants from the National Natural Science Foundation of China (grant number 82072114) and the Fundamental Research Funds for the Central Universities of Central South University (2023ZZTS0546).
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by Jiao **ao. **angyan Zhang contributed to conception and design and carried out the analysis and interpretation of data. Fengqin Yang and Hongke Qu revised the draft and approved the revisions. Chengxin Ye, Sile Chen performed acquisition and analysis and interpretation of data. Supervision: Yadong Guo. All authors approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Informed consent
Written informed consent for publishing this scientific report was obtained from the direct relative of the decedent in this case.
Conflict of interest
The authors declare no competing interests.
Ethics approval
The study was approved by the Ethics Committee of Central South University and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
**angyan Zhang and Jiao **ao are co-first authors and they made equal contributions to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., **ao, J., Yang, F. et al. Identification of sudden cardiac death from human blood using ATR-FTIR spectroscopy and machine learning. Int J Legal Med 138, 1139–1148 (2024). https://doi.org/10.1007/s00414-023-03118-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-023-03118-7