Abstract
Background
Poor ovarian response (POR) is associated with decreased clinical pregnancy rates, emphasizing the need for develo** clinical prediction models. Such models can improve prognostic accuracy, personalize medical interventions, and ultimately enhance live birth rates among patients with POR.
Objective
This study aims to develop and validate a prognostic model for predicting clinical pregnancy outcomes in individuals with POR undergoing in vitro fertilization/ intracytoplasmic sperm injection (IVF/ICSI) cycles.
Methods
A retrospective cohort of 969 patients with POR undergoing fresh embryo transfer cycles at the Reproductive Center of Fujian Maternal and Child Health Center from January 2018 to January 2022 was included. The cohort was randomly divided into model (n = 678) and validation (n = 291) groups in a 7:3 ratio. A single-factor analysis was performed on the model group to identify variables influencing clinical pregnancy. Optimal variables were selected using LASSO regression, and a clinical prediction model was constructed using multivariate logistic regression analysis. The model's calibration and discrimination were assessed using receiver operating characteristic (ROC) and calibration curves, while the clinical utility was evaluated using decision curve analysis.
Results
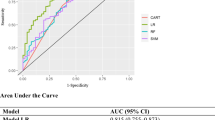
Multivariate logistic regression analysis revealed that the age of the women (odds ratio [OR] 0.936, 95% confidence interval [CI] 0.898–0.976, P = 0.002), body mass index (BMI) ≤ 24 (OR 2.748, 95% CI 1.724–4.492, P < 0.001), antral follicle count (AFC) (OR 1.232, 95% CI 1.073–1.416, P = 0.003), anti-Müllerian hormone (AMH) (OR 1.67, 95% CI 1.178–2.376, P = 0.004), number of mature oocytes (OR 1.227, 95% CI 1.075–1.403, P = 0.003), number of embryos transferred (OR 1.692, 95% CI 1.132–2.545, P = 0.011), and transfer of high-quality embryos (OR 3.452, 95% CI 1.548–8.842, P = 0.005) were independent predictors of clinical pregnancy in patients with POR. According to the receiver operating characteristic (ROC) analysis, the prediction model exhibited an area under the curve (AUC) of 0.752 (0.714, 0.789) in the model group and 0.765 (0.708, 0.821) in the validation group. The clinical decision curve demonstrated that the model held maximum clinical utility in both cohorts when the threshold probability of clinical pregnancy ranged from 6–81% to 12–82%, respectively.
Conclusion
Clinical pregnancy outcomes in patients with POR who underwent IVF/ICSI treatment were influenced by several independent factors, including the age of the women, BMI, AFC, AMH, number of mature oocytes, number of embryos transferred, and transfer of high-quality embryos. A clinical prediction model based on these factors exhibited favorable clinical predictive and applicative value. Therefore, this model can serve as a valuable tool for clinical prognosis, intervention, and facilitating personalized medical treatment.






Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
References
Vaiarelli A, Cimadomo D, Ubaldi N et al (2018) What is new in the management of poor ovarian response in IVF? Curr Opin Obstet Gynecol 30(3):155–162. https://doi.org/10.1097/GCO.0000000000000452
Qiao J, Ma CH, Liu JY et al (2015) A consensus of poor ovarian response. Reprod Contracep 35(4):211–223. https://doi.org/10.7669/j.issn.0253-357X.2015.04.0211
Ferraretti AP, La Marca A, Fauser BC et al (2011) ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 26(7):1616–1624. https://doi.org/10.1093/humrep/der092
Alviggi C, Andersen CY, Buehler K et al (2016) A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril 105(6):1452–1453. https://doi.org/10.1016/j.fertnstert.2016.02.005
Gong X, Zhang Y, Zhu Y, et al. Development and validation of a live birth prediction model for expected poor ovarian response patients during IVF/ICSI.Front Endocrinol (Lausanne). 2023, 31(14):1027805. https://doi.org/10.3389/fendo.2023.1027805.
Conforti A, Tüttelmann F, Alviggi C et al (2021) Effect of genetic variants of gonadotropins and their receptors on ovarian stimulation outcomes: a Delphi consensus. Front Endocrinol (Lausanne) 12:797365. https://doi.org/10.3389/fendo.2021.797365
Esteves SC, Alviggi C, Humaidan P et al (2019) The POSEIDON criteria and its measure of success through the eyes of clinicians and embryologists. Front Endocrinol (Lausanne) 10:814. https://doi.org/10.3389/fendo.2019.00814
Lundin K, Ahlström A (2015) Quality control and standardization of embryo morphology scoring and viability markers. Reprod Biomed Online 31(4):459–471. https://doi.org/10.1016/j.rbmo.2015.06.026
Gardner DK, Lane M, Stevens J et al (2000) Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 73(6):1155–1158. https://doi.org/10.1016/s0015-0282(00)00518-5
Cedars MI (2022) Managing poor ovarian response in the patient with diminished ovarian reserve. Fertil Steril 117(4):655–656. https://doi.org/10.1016/j.fertnstert.2022.02.026
Grisendi V, Mastellari E, La Marca A (2019) Ovarian reserve markers to identify poor responders in the context of poseidon classification. Front Endocrinol (Lausanne) 10:281. https://doi.org/10.3389/fendo.2019.00281
Wu XQ, Kong R, Tian L et al (2015) A consensus of poor ovarian response. Reprod Contracep 35(2):71–79. https://doi.org/10.7669/j.issn.0253-357X.2015.02.0071
Lebovitz O, Haas J, Mor N et al (2022) Predicting IVF outcome in poor ovarian responders. BMC Womens Health 22(1):395. https://doi.org/10.1186/s12905-022-01964-y
Fischer R, Baukloh V (2020) Commentary: management strategies for POSEIDON Groups 3 and 4. Front Endocrinol (Lausanne) 11:34. https://doi.org/10.3389/fendo.2020.00034
Shen YJ, Deng XH, Yu HL et al (2020) A clinical model for predicting the resuscitation cycle of single blastocyst transplantation. Prog Modern Obstet Gynecol 29(3):194–198. https://doi.org/10.13283/j.cnki.xdfckjz.2020.03.031
Du Y, Chen L, Lin J et al (2018) Chromosomal karyotype in chorionic villi of recurrent spontaneous abortion patients. Biosci Trends 12(1):32–39. https://doi.org/10.5582/bst.2017.01296
Chen Y, Bartanus J, Liang D et al (2017) Characterization of chromosomal abnormalities in pregnancy losses reveals critical genes and loci for human early development. Hum Mutation 38(6):669–677. https://doi.org/10.1002/humu.23207
Goldman RH, Farland LV, Thomas AM et al (2019) The combined impact of maternal age and body mass index on cumulative live birth following in vitro fertilization. Am J Obstet Gynecol 221:617.e1-617.e13. https://doi.org/10.1016/j.ajog.2019.05.043
Bleil ME, Gregorich SE, Adler NE et al (2014) Race/ethnic disparities in reproductive age: an examination of ovarian reserve estimates across four race/ethnic groups of healthy, regularly cycling women. Fertil Steril 101(1):199–207. https://doi.org/10.1016/j.fertnstert.2013.09.015
Yin HQ, Jiang H, He RB et al (2019) Cumulative live birth rate of advanced-age women more than 40 with or without poor ovarian response. Taiwan J Obstet Gynecol 58:201–205. https://doi.org/10.1016/j.tjog.2019.01.006
Scheffer JB, Scheffer BB, de Carvalho RF et al (2017) Age as a predictor of embryo quality regardless of the quantitative ovarian response. Int J Fertil Steril 11:40–46. https://doi.org/10.22074/ijfs.2016.4579
Chang MY, Chiang CH, Hsieh TT et al (1998) Use of the antral follicle count to predict the outcome of assisted reproductive technologies. Fertil Steril 69:505–510. https://doi.org/10.1016/s0015-0282(97)00557-8
Polyzos NP, Popovic-Todorovic B (2020) Say No to mild ovarian stimulation for all poor responders: it is time to realize that not all poor responders are the same. Hum Reprod 35(9):1964–1971. https://doi.org/10.1093/humrep/deaa183
Hochberg A, Dahan MH, Yarali H et al (2024) Significance of serum AMH and antral follicle count discrepancy for the prediction of ovarian stimulation response in Poseidon criteria patients. J Assist Reprod Genet. https://doi.org/10.1007/s10815-024-03050-8
Albert H, Margaret A, Knee Alexander B et al (2011) Antral follicle count in clinical practice: analyzing clinical relevance. Fertil Steril 95:474–479. https://doi.org/10.1016/j.fertnstert.2010.03.023
Broer SL, Dólleman M, Opmeer BC et al (2011) AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update 17(1):46–54. https://doi.org/10.1093/humupd/dmq034
McCallie BR, Haywood M, Denomme MM et al (2021) Forecasting early onset diminished ovarian reserve for young reproductive age women. J Assist Reprod Genet 38(7):1853–1860. https://doi.org/10.1007/s10815-021-02155-8
Dewailly D, Laven J (2019) AMH as the primary marker for fertility. Eur J Endocrinol 181(6):D45–D51. https://doi.org/10.1530/EJE-19-0373
Guo Y, Jiang H, Hu S et al (2021) Efficacy of three COS protocols and predictability of AMH and AFC in women with discordant ovarian reserve markers: a retrospective study on 19,239 patients. J Ovarian Res 14(1):111. https://doi.org/10.1186/s13048-021-00863-4
Shrikhande L, Shrikhande B, Shrikhande A (2020) AMH and its clinical implications. J Obstet Gynaecol India 70(5):337–341. https://doi.org/10.1007/s13224-020-01362-0
Sermondade N, Huberlant S, Bourhis-Lefebvre V et al (2019) Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update 25:439–451
Druzhinina AS, Vitiazeva II, Dimitrova DA (2021) Correlation of in vitro fertilization (IVF) infertility treatment outcomes and body weight index in women of reproductive age. Probl Endokrinol (Mosk) 67:76–82
Valent AM, Hall ES, Defranco EA (2016) The influence of obesity on perinatal outcomes in pregnancies achieved with assisted reproductive technology: a population-based retrospective cohort study. Obstet Med 9(1):34–39. https://doi.org/10.1177/1753495X15621152
Malchau SS, Henningsen AA, Forman J et al (2019) Cumulative live birth rate prognosis based on the number of aspirated oocytes in previous ART cycles. Hum Reprod 34(1):171–180. https://doi.org/10.1093/humrep/dey341
Stanger JD, Yovich JL (2013) Follicle recruitment determines IVF productivity rate via the number of embryos frozen and subsequent transfers. Reprod Biomed Online 27(3):286–296. https://doi.org/10.1016/j.rbmo.2013.05.015
Brodin T, Hadziosmanovic N, Berglund L et al (2015) Comparing four ovarian reserve markers–associations with ovarian response and live births after assisted reproduction. Acta Obstet Gynecol Scand 94(10):1056–1063. https://doi.org/10.1111/aogs.12710
Funding
This study received support from the Innovation Platform Project of Science and Technology, Fujian Province (2021Y2012), the Key Project on the Integration of Industry, Education and Research Collaborative Innovation of Fujian Province (grant no. 2021YZ034011), the Key Project on the Science and Technology Program of Fujian Health Commission (grant no. 2021ZD01002), the Fujian Provincial Health Technology Project (no. 2022GGA035), the Major Scientific Research Program for Young and Middle-aged Health Professionals of Fujian Province, China (grant no. 2022ZQNZD010), and the Natural Science Foundation of Fujian Province (grant no. 2023J011221).
Author information
Authors and Affiliations
Contributions
CXJ and ZBH conceived and supervised the study. CLL and JWW collected patient data. ZSQ, LRS, and JWW analyzed and interpreted the data. ZSQ, SY, and ZBH contributed to manuscript development. All authors have reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, S., Jiang, W., Sun, Y. et al. Nomogram to predict the probability of clinical pregnancy in women with poor ovarian response undergoing in vitro fertilization/ intracytoplasmic sperm injection cycles. Arch Gynecol Obstet (2024). https://doi.org/10.1007/s00404-024-07598-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00404-024-07598-9