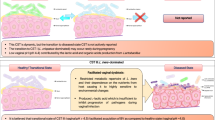
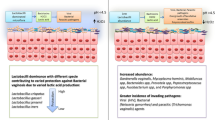
Abstract
Vaginal canal (VC) is exposed to the external environment affected by habitual factors like hygiene and sexual behaviour as well as physiological factors like puberty, menstrual cycle, pregnancy, child birth and menopause. Healthy VC harbours beneficial microflora supported by vaginal epithelium and cervical fluid. Connatural antimicrobial peptide (AMPs) of female reproductive tract (FRT) conjunctly with these beneficial microbes provide protection from a large number of infectious diseases. Such infections may either be caused by native microbes of the VC or transitory microbes like bacteria or virus which are not a part of VC microflora. This review highlight’s the role of hormones, enzymes, innate immunological factors, epithelial cells and vaginal mucus that support beneficial microbes over infectious ones thus, hel** to maintain homeostasis in VC and further protect the FRT. We also discuss the prospective use of vaginal probiotics and AMPs against pathogens which can serve as a potential cure for vaginal infections.
Similar content being viewed by others
References
Priestley CJ, Jones BM, Dhar J, Goodwin L (1997) What is normal vaginal flora? Sex Transmit Infect 73(1):23–28
Martin HL Jr, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J (1999) Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 180(6):1863–1868
Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, Frechette GM, Hill ET, Fahey JV (2011) Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol 4(3):335–342
Monin L, Whettlock EM, Male V (2020) Immune responses in the human female reproductive tract. Immunology 160(2):106–115
Nasu K, Narahara H (2010) Pattern recognition via the toll-like receptor system in the human female genital tract. Med Inflam 2010:1
Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA (1993) The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis 16(Supplement_4):S273–S281
Graver MA, Wade JJ (2011) The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann Clin Microbiol Antimicrob 10(1):1–5
Molenaar MC, Singer M, Ouburg S (2018) The two-sided role of the vaginal microbiome in Chlamydia trachomatis and Mycoplasma genitalium pathogenesis. J Reprod Immunol 130:11–17
Mitchell C, Marrazzo J (2014) Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol 71(6):555–563
Bartlett JG, Polk BF (1984) Bacterial flora of the vagina: quantitative study. Rev Infect Dis 6(Supplement_1):S67-72
Abdool Karim SS, Baxter C, Passmore JA, McKinnon LR, Williams BL (2019) The genital tract and rectal microbiomes: their role in HIV susceptibility and prevention in women. J Int AIDS Soc 22(5):e25300
Corbishley CM (1977) Microbial flora of the vagina and cervix. J Clin Pathol 30(8):745–748
Gregoire AT, Kandil O, Ledger WJ (1971) The glycogen content of human vaginal epithelial tissue. Fertil Steril 22(1):64–68
Hammerschlag MR, Alpert S, Onderdonk AB, Thurston P, Drude E, McCormack WM, Bartlett JG (1978) Anaerobic microflora of the vagina in children. Am J Obstet Gynecol 131(8):853–856
Farage M, Maibach H (2006) Lifetime changes in the vulva and vagina. Arch Gynecol Obstet 273(4):195–202
Hammerschlag MR, Alpert S, Rosner I, Thurston P, Semine D, McComb D, McCormack WM (1978) Microbiology of the vagina in children: normal and potentially pathogenic organisms. Pediatrics 62(1):57–62
Wessels JM, Felker AM, Dupont HA, Kaushic C (2018) The relationship between sex hormones, the vaginal microbiome and immunity in HIV-1 susceptibility in women. Dis Models Mech 11(9):dmm035147
Gregoire AT, Parakkal PF (1972) Glycogen content in the vaginal tissue of normally cycling and estrogen and progesterone-treated rhesus monkeys. Biol Reprod 7(1):9–14
Nasioudis D, Beghini J, Bongiovanni AM, Giraldo PC, Linhares IM, Witkin SS (2015) α-Amylase in vaginal fluid: association with conditions favorable to dominance of Lactobacillus. Reprod Sci 22(11):1393–1398
Spear GT, French AL, Gilbert D, Zariffard MR, Mirmonsef P, Sullivan TH, Spear WW, Landay A, Micci S, Lee BH, Hamaker BR (2014) Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis 210(7):1019–1028
Mirmonsef P, Gilbert D, Zariffard MR, Hamaker BR, Kaur A, Landay AL, Spear GT (2011) The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol 65(3):190–195
Tachedjian G, Aldunate M, Bradshaw CS, Cone RA (2017) The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol 168(9–10):782–792
Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S (2001) Defense factors of vaginal lactobacilli. Am J Obstet Gynecol 185(2):375–379
Anderson DJ, Marathe J, Pudney J (2014) The structure of the human vaginal stratum corneum and its role in immune defense. Am J Reprod Immunol 71(6):618–623
Katz DF (1991) Human cervical mucus: research update. Am J Obstet Gynecol 165(6):1984–1986
Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UM, Zhong X, Koenig SS, Fu L, Ma Z, Zhou X, Abdo Z (2012) Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4(132):132ra52
Zhang H, Feng Q, Zhu Z, Dai H, Hu H (2021) The value of vaginal microbiome in patients with endometrial hyperplasia. J Healthc Eng 2021:1
Lee SK, Kim CJ, Kim DJ, Kang JH (2015) Immune cells in the female reproductive tract. Immune network 15(1):16–26
Moreno I, Codoñer FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazán J, Alonso R, Alamá P, Remohí J, Pellicer A, Ramon D (2017) Evidence that the endometrial microbiota has an effect on implantation success or failure. Obstet Gynecol Surv 72(6):341–342
Altmäe S (2018) Commentary: uterine microbiota: Residents, tourists, or invaders? Front Immunol 1874:1
Keane FE, Ison CA, Taylor-Robinson D (1997) A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD AIDS 8(8):489–494
Witkin SS, Linhares IM, Giraldo P (2007) Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynaecol 21(3):347–354
Kervinen K, Kalliala I, Glazer-Livson S, Virtanen S, Nieminen P, Salonen A (2019) Vaginal microbiota in pregnancy: Role in induction of labor and seeding the neonate’s microbiota? J Biosci 44(5):1–6
Pelzer ES, Allan JA, Theodoropoulos C, Ross T, Beagley KW, Knox CL (2012) Hormone-dependent bacterial growth, persistence and biofilm formation—a pilot study investigating human follicular fluid collected during IVF cycles. PLoS ONE 7(12):e49965
Ishida T, Seo F, Hirato K, Fukuda T, Yanaihara T, Araki H, Nakayama T (1985) Changes in placental enzymatic activities in relation to estrogen production during pregnancy. Nihon Sanka Fu**ka Gakkai Zasshi 37(4):547–554
Pretorius C, Jagatt A, Lamont RF (2007) The relationship between periodontal disease, bacterial vaginosis, and preterm birth. J Perinat Med 35(2):93–99. https://doi.org/10.1515/JPM.2007.039
Pararas MV, Skevaki CL, Kafetzis DA (2006) Preterm birth due to maternal infection: causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis 25(9):562–569
Elovitz M, Gajer P, Bastek J, Anglim L, Brown A, Ravel J (2014) 26: the cervicovaginal microbiota is different in women destined to have a preterm birth. Am J Obstet Gynecol 210(1):S16–S17
Prince AL, Chu DM, Seferovic MD, Antony KM, Ma J, Aagaard KM (2015) The perinatal microbiome and pregnancy: moving beyond the vaginal microbiome. Cold Spring Harb Perspect Med 5(6):a023051
Shi YC, Guo H, Chen J, Sun G, Ren RR, Guo MZ, Peng LH, Yang YS (2018) Initial meconium microbiome in Chinese neonates delivered naturally or by cesarean section. Sci Rep 8(1):1–2
Walker RW, Clemente JC, Peter I, Loos RJ (2017) The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr Obes 12:3–17
Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG (2015) The infant microbiome development: mom matters. Trends Mol Med 21(2):109–117
Caillouette JC, Sharp CF Jr, Zimmerman GJ, Roy S (1997) Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol 176(6):1270–1277
Murta EF, Barcelos A (2005) Relation between vaginal and endocervical pH in pre-and post-menopausal women. Arch Gynecol Obstet 272(3):211–213
Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver M, Viscidi RP, Burke AE, Ravel J, Gravitt PE (2014) Association between the vaginal microbiota, menopause status and signs of vulvovaginal atrophy. Menopause (New York) 21(5):450
Devillard E, Burton JP, Hammond JA, Lam D, Reid G (2004) Novel insight into the vaginal microflora in postmenopausal women under hormone replacement therapy as analyzed by PCR-denaturing gradient gel electrophoresis. Eur J Obstet Gynecol Reprod Biol 117(1):76–81
Petricevic L, Domig KJ, Nierscher FJ, Krondorfer I, Janitschek C, Kneifel W, Kiss H (2012) Characterisation of the oral, vaginal and rectal Lactobacillus flora in healthy pregnant and postmenopausal women. Eur J Obstet Gynecol Reprod Biol 160(1):93–99
Akimoto-Gunther L, Bonfim-Mendonca PD, Takahachi G, Irie MM, Miyamoto S, Consolaro ME, Svidzinsk TI (2016) Highlights regarding host predisposing factors to recurrent vulvovaginal candidiasis: chronic stress and reduced antioxidant capacity. PLoS ONE 11(7):e0158870
Amabebe E, Anumba DO (2018) Psychosocial stress, cortisol levels, and maintenance of vaginal health. Front Endocrinol 568:1
Belay T, Woart A (2013) Cold-induced stress increases the intensity of Chlamydia genital infection in mice. J Microbiol Immunol Infect 46(5):330–337
Borgogna JL, Anastario M, Firemoon P, Rink E, Ricker A, Ravel J, Brotman RM, Yeoman CJ (2021) Vaginal microbiota of American Indian women and associations with measures of psychosocial stress. PLoS ONE 16(12):e0260813
Borges S, Silva J, Teixeira P (2014) The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet 289(3):479–489
George F, Daniel C, Thomas M, Singer E, Guilbaud A, Tessier FJ, Revol-Junelles AM, Borges F, Foligné B (2018) Occurrence and dynamism of lactic acid bacteria in distinct ecological niches: a multifaceted functional health perspective. Front Microbiol 2899:1
Wang H, Ma Y, Li R, Chen X, Wan L, Zhao W (2019) Associations of cervicovaginal lactobacilli with high-risk human papillomavirus infection, cervical intraepithelial neoplasia, and cancer: a systematic review and meta-analysis. J Infect Dis 220(8):1243–1254
Mitra A, MacIntyre DA, Lee YS, Smith A, Marchesi JR, Lehne B, Bhatia R, Lyons D, Paraskevaidis E, Li JV, Holmes E (2015) Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep 5(1):1–1
Mancabelli L, Tarracchini C, Milani C, Lugli GA, Fontana F, Turroni F, van Sinderen D, Ventura M (2021) Vaginotypes of the human vaginal microbiome. Environ Microbiol 23(3):1780–1792
Song SD, Acharya KD, Zhu JE, Deveney CM, Walther-Antonio MR, Tetel MJ, Chia N (2020) Daily vaginal microbiota fluctuations associated with natural hormonal cycle, contraceptives, diet, and exercise. Msphere 5(4):e00593-e620
Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, Galuppi M, Lamont RF, Chaemsaithong P, Miranda J, Chaiworapongsa T (2014) The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2(1):1–9
Smith SB, Ravel J (2017) The vaginal microbiota, host defence and reproductive physiology. J Physiol 595(2):451–463
Ahire JJ, Sahoo S, Kashikar MS, Heerekar A, Lakshmi SG, Madempudi RS (2021) In vitro assessment of Lactobacillus crispatus UBLCp01, Lactobacillus gasseri UBLG36, and Lactobacillus johnsonii UBLJ01 as a potential vaginal probiotic candidate. Probiot Antimicrob Proteins 20:1–2
Witkin SS, Linhares IM (2017) Why do lactobacilli dominate the human vaginal microbiota? BJOG Int J Obstet Gynaecol 124(4):606–611
Vaneechoutte M (2017) Lactobacillus iners, the unusual suspect. Res Microbiol 168(9–10):826–836
Gerson KD, McCarthy C, Elovitz MA, Ravel J, Sammel MD, Burris HH (2020) Cervicovaginal microbial communities deficient in Lactobacillus species are associated with second trimester short cervix. Am J Obstet Gynecol 222(5):491-e1
Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ (2013) Influence of vaginal bacteria and D-and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. MBio 4(4):e00460-13
Eastment MC, McClelland RS (2018) Vaginal microbiota and susceptibility to HIV. AIDS (London) 32(6):687
van Houdt R, Ma B, Bruisten SM, Speksnijder AG, Ravel J, de Vries HJ (2018) Lactobacillus iners-dominated vaginal microbiota is associated with increased susceptibility to Chlamydia trachomatis infection in Dutch women: a case–control study. Sex Transmit Infect 94(2):117–23
Albert AY, Chaban B, Wagner EC, Schellenberg JJ, Links MG, Van Schalkwyk J, Reid G, Hemmingsen SM, Hill JE, Money D, VOGUE Research Group (2015) A study of the vaginal microbiome in healthy Canadian women utilizing cpn 60-based molecular profiling reveals distinct Gardnerella subgroup community state types. PLoS ONE 10(8):0135620
Boskey ER, Cone RA, Whaley KJ, Moench TR (2001) Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod 16(9):1809–13
Amabebe E, Anumba DO (2018) The vaginal microenvironment: the physiologic role of lactobacilli. Front Med 5:181
Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M (2009) Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol 9(1):1
Beghini J, Linhares IM, Giraldo PC, Ledger WJ, Witkin SS (2015) Differential expression of lactic acid isomers, extracellular matrix metalloproteinase inducer, and matrix metalloproteinase-8 in vaginal fluid from women with vaginal disorders. BJOG Int J Obstet Gynaecol 122(12):1580–5
Witkin SS (2015) The vaginal microbiome, vaginal anti-microbial defence mechanisms and the clinical challenge of reducing infection-related preterm birth. BJOG Int J Obstet Gynaecol 122(2):213–8
Hearps AC, Tyssen D, Srbinovski D, Bayigga L, Diaz DJ, Aldunate M, Cone RA, Gugasyan R, Anderson DJ, Tachedjian G (2017) Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol 10(6):1480–90
Joo HM, Hyun YJ, Myoung KS, Ahn YT, Lee JH, Huh CS, Han MJ, Kim DH (2011) Lactobacillus johnsonii HY7042 ameliorates Gardnerella vaginalis-induced vaginosis by killing Gardnerella vaginalis and inhibiting NF-κB activation. Int Immunopharmacol 11(11):1758–65
Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, Holmes KK (1989) Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol 27(2):251–6
Hillier SL, Krohn MA, Klebanoff SJ, Eschenbach DA (1992) The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women. Obstet Gynecol 79(3):369–73
O’Hanlon DE, Moench TR, Cone RA (2011) In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect Dis 11(1):1–8
Tomás MS, Otero MC, Ocaña V, Nader-Macías ME (2004) Production of antimicrobial substances by lactic acid bacteria I. In public health microbiology. Humana Press, London, pp 337–346
Florou-Paneri P, Christaki E, Bonos E (2013) Lactic acid bacteria as source of functional ingredients InLactic acid bacteria-R&D for food, health and livestock purposes. IntechOpen 1:1
Howett MK, Kuhl JP (2005) Microbicides for prevention of transmission of sexually transmitted diseases. Curr Pharm Des 11(29):3731–46
Nelson J, El-Gendy AO, Mansy MS, Ramadan MA, Aziz RK (2020) The biosurfactants iturin, lichenysin and surfactin, from vaginally isolated lactobacilli, prevent biofilm formation by pathogenic Candida. FEMS Microbiol Lett 367(15):fnaa126
Zárate G, Nader-Macias ME (2006) Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett Appl Microbiol 43(2):174–80
Naidu AS, Bidlack WR, Clemens RA (1999) Probiotic spectra of lactic acid bacteria (LAB). Crit Rev Food Sci Nutr 39(1):13–26
Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y, Li L, Nelson KE, **a Y, **ang C (2010) Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics 11(1):1–6
Beigi RH, Wiesenfeld HC, Hillier SL, Straw T, Krohn MA (2005) Factors associated with absence of H2O2-producing Lactobacillus among women with bacterial vaginosis. J Infect Dis 191(6):924–9
Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wølner-Hanssen P, Holmes KK (1996) Hydrogen peroxide—producing lactobacilli and acquisition of vaginal infections. J Infect Dis 174(5):1058–63
Spiegel CA (2002) Bacterial vaginosis. Rev Med Microbiol 13(2):43–51
Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, Marrazzo JM, Fredricks DN (2010) Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS ONE 5(4):e10197
Onderdonk AB, Delaney ML, Fichorova RN (2016) The human microbiome during bacterial vaginosis. Clin Microbiol Rev 29(2):223–38
Wiggins R, Hicks SJ, Soothill PW, Millar MR, Corfield AP (2001) Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex Transmit Infect 77(6):402–8
Gondo DC, Duarte MT, Silva MG, Parada CM (2010) Abnormal vaginal flora in low-risk pregnant women cared for by a public health service: prevalence and association with symptoms and findings from gynecological exams. Rev Lat Am Enfermagem 18:919–27
Castro J, Martins AP, Rodrigues ME, Cerca N (2018) Lactobacillus crispatus represses vaginolysin expression by BV associated Gardnerella vaginalis and reduces cell cytotoxicity. Anaerobe 50:60–3
McGregor JA, French JI, Jones W, Milligan K, McKinney PJ, Patterson E, Parker R (1994) Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase: results of a controlled trial of topical clindamycin cream. Am J Obstet Gynecol 170(4):1048–60
Li W, Yang S, Kim SO, Reid G, Challis JR, Bocking AD (2014) Lipopolysaccharide-induced profiles of cytokine, chemokine, and growth factors produced by human decidual cells are altered by Lactobacillus rhamnosus GR-1 supernatant. Reprod Sci 21(7):939–47
Valore EV, Wiley DJ, Ganz T (2006) Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun 74(10):5693–702
Libby EK, Pascal KE, Mordechai E, Adelson ME, Trama JP (2008) Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes Infect 10(4):439–46
Donders GG, Bellen G, Rezeberga D (2011) Aerobic vaginitis in pregnancy. BJOG Int J Obstet Gynaecol 118(10):1163–70
Tansarli GS, Kostaras EK, Athanasiou S, Falagas ME (2013) Prevalence and treatment of aerobic vaginitis among non-pregnant women: evaluation of the evidence for an underestimated clinical entity. Eur J Clin Microbiol Infect Dis 32(8):977–84
Donders GG, Bellen G, Grinceviciene S, Ruban K, Vieira-Baptista P (2017) Aerobic vaginitis: no longer a stranger. Res Microbiol 168(9–10):845–58
Shiroda M, Aronoff DM, Gaddy JA, Manning SD (2020) The impact of Lactobacillus on group B streptococcal interactions with cells of the extraplacental membranes. Microb Pathog 148:104463
Shazadi K, Ahmad SZ, Ahmad SS, Arshad N (2021) In vivo prophylactic efficacy of Lactobacillus reuteri MT180537 against aerobic vaginitis. Microb Pathog 160:105197
Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, Szell M, Dobozy A, Kemeny L (2005) Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human β-defensin-2 in vaginal epithelial cells. Microbes Infect 7(9–10):1117–27
Bertuccini L, Russo R, Iosi F, Superti F (2017) Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int J Immunopathol Pharmacol 30(2):163–7
Spiegel CA, Amsel R, Eschenbach D, Schoenknecht F, Holmes KK (1980) Anaerobic bacteria in nonspecific vaginitis. N Engl J Med 303(11):601–7
Odds FC (1994) Pathogenesis of Candida infections. J Am Acad Dermatol 31(3):S2-5
Er S, Tosun Aİ, Berk GA, Kivanc M (2019) Anticandidal activities of lactic acid bacteria isolated from the vagina. Turk J Med Sci 49(1):375–83
De Repentigny L, Aumont F, Bernard K, Belhumeur P (2000) Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect Immun 68(6):3172–9
MacAlpine J, Daniel-Ivad M, Liu Z, Yano J, Revie NM, Todd RT, Stogios PJ, Sanchez H, O’Meara TR, Tompkins TA, Savchenko A (2021) A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase. Nat Commun 12(1):1–6
Kalo-Klein A, Witkin SS (1989) Candida albicans: cellular immune system interactions during different stages of the menstrual cycle. Am J Obstet Gynecol 161(5):1132–6
Gupta K, Hillier SL, Hooton TM, Roberts PL, Stamm WE (2000) Effects of contraceptive method on the vaginal microbial flora: a prospective evaluation. J Infect Dis 181(2):595–601
Abdul-Aziz M, Mahdy MA, Abdul-Ghani R, Alhilali NA, Al-Mujahed LK, Alabsi SA, Al-Shawish FA, Alsarari NJ, Bamashmos W, Abdulwali SJ, Al KM (2019) Bacterial vaginosis, vulvovaginal candidiasis and trichomonal vaginitis among reproductive-aged women seeking primary healthcare in Sana’a city. Yemen BMC Infect Dis 19(1):1
Hooton TM, Fennell CL, Clark AM, Stamm WE (1991) Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J Infect Dis 164(6):1216–9
Achilles SL, Hillier SL (2013) The complexity of contraceptives: understanding their impact on genital immune cells and vaginal microbiota. AIDS (London) 27(01):S5
Fichorova RN, Chen PL, Morrison CS, Doncel GF, Mendonca K, Kwok C, Chipato T, Salata R, Mauck C (2015) The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio 6(5):e00221-15
Liu CM, Hungate BA, Tobian AA, Serwadda D, Ravel J, Lester R, Kigozi G, Aziz M, Galiwango RM, Nalugoda F, Contente-Cuomo TL (2013) Male circumcision significantly reduces prevalence and load of genital anaerobic bacteria. MBio 4(2):e00076-13
Marrazzo JM, Fiedler TL, Srinivasan S, Thomas KK, Liu C, Ko D, **e H, Saracino M, Fredricks DN (2012) Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis 205(10):1580–8
Breshears LM, Edwards VL, Ravel J, Peterson ML (2015) Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol 15(1):1–2
Coleman JS, Gaydos CA, Witter F (2013) Trichomonas vaginalis vaginitis in obstetrics and gynecology practice: new concepts and controversies. Obstet Gynecol Surv 68(1):43
Brotman RM, Bradford LL, Conrad M, Gajer P, Ault K, Peralta L, Forney LJ, Carlton JM, Abdo Z, Ravel J (2012) Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis 39(10):807
Witkin SS, Minis E, Athanasiou A, Leizer J, Linhares IM (2017) Chlamydia trachomatis: the persistent pathogen. Clin Vaccine Immunol 24(10):e00203-17
Hafner LM (2015) Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections. Contraception 92(2):108–15
Ziklo N, Huston WM, Hocking JS, Timms P (2016) Chlamydia trachomatis genital tract infections: when host immune response and the microbiome collide. Trends Microbiol 24(9):750–65
Parolin C, Frisco G, Foschi C, Giordani B, Salvo M, Vitali B, Marangoni A, Calonghi N (2018) Lactobacillus crispatus BC5 interferes with Chlamydia trachomatis infectivity through integrin modulation in cervical cells. Front Microbiol 2630:1
Hill SA, Masters TL, Wachter J (2016) Gonorrhea—an evolving disease of the new millennium. Microbial cell 3(9):371
Foschi C, Salvo M, Cevenini R, Parolin C, Vitali B, Marangoni A (2017) Vaginal lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: an in vitro study. Front Cell Infect Microbiol 7:502
Pereira N, Kucharczyk KM, Estes JL, Gerber RS, Lekovich JP, Elias RT, Spandorfer SD (2015) Human papillomavirus infection, infertility, and assisted reproductive outcomes. J Pathogens 2015:1
Platz-Christensen JJ, Sundström E, Larsson PG (1994) Bacterial vaginosis and cervical intraepithelial neoplasia. Acta Obstet Gynecol Scand 73(7):586–8
Ribelles P, Benbouziane B, Langella P, Suárez JE, Bermúdez-Humarán LG, Riazi A (2013) Protection against human papillomavirus type 16-induced tumors in mice using non-genetically modified lactic acid bacteria displaying E7 antigen at its surface. Appl Microbiol Biotechnol 97(3):1231–9
Cortes-Perez NG, Kharrat P, Langella P, Bermúdez-Humarán LG (2009) Heterologous production of human papillomavirus type-16 L1 protein by a lactic acid bacterium. BMC Res Notes 2(1):1–8
Gupta R, Warren T, Wald A (2007) Genital herpes. Lancet 370(9605):2127–37
Masese L, Baeten JM, Richardson BA, Bukusi E, John-Stewart G, Jaoko W, Shafi J, Kiarie J, McClelland RS (2014) Incident herpes simplex virus type 2 infection increases the risk of subsequent episodes of bacterial vaginosis. J Infect Dis 209(7):1023–7
Conti C, Malacrino C, Mastromarino P (2009) Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J Physiol Pharmacol 60(Suppl 6):19–26
Kassaa IA, Hober D, Hamze M, Caloone D, Dewilde A, Chihib NE, Drider D (2015) Vaginal Lactobacillus gasseri CMUL57 can inhibit herpes simplex type 2 but not Coxsackievirus B4E2. Arch Microbiol 197(5):657–64
Bayigga L, Kateete DP, Anderson DJ, Sekikubo M, Nakanjako D (2019) Diversity of vaginal microbiota in sub-Saharan Africa and its effects on HIV transmission and prevention. Am J Obstet Gynecol 220(2):155–66
Soloyan H, De Filippo RE, Sedrakyan S. Tissue engineering of the reproductive system
İzgü F, Bayram G, Tosun K, İzgü D (2017) Stratum corneum lipid liposome-encapsulated panomycocin: Preparation, characterization, and the determination of antimycotic efficacy against Candida spp. isolated from patients with vulvovaginitis in an in vitro human vaginal epithelium tissue model. Int J Nanomed 12:5601
Davis ME, Hartman CG (1935) Changes in vaginal epithelium during pregnancy in relation to the vaginal cycle. J Am Med Assoc 104(4):279–85
Boris S, Suárez JE, Vázquez F, Barbés C (1998) Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect Immun 66(5):1985–1989
Wira CR, Grant-Tschudy KS, Crane-Godreau MA (2005) Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol 53(2):65–76
Rutllant J, López-Béjar M, López-Gatius F (2005) Ultrastructural and rheological properties of bovine vaginal fluid and its relation to sperm motility and fertilization: a review. Reprod Domest Anim 40(2):79–86
Valore EV, Park CH, Igreti SL, Ganz T (2002) Antimicrobial components of vaginal fluid. Am J Obstet Gynecol 187(3):561–8
Govers J, Girard JP (1972) Some immunological properties of human cervical and vaginal secretions. Gynecol Obstet Invest 3(5–6):184–94
Adnane M, Meade KG, O’Farrelly C (2018) Cervico-vaginal mucus (CVM)—an accessible source of immunologically informative biomolecules. Vet Res Commun 42(4):255–63
Sivaran**i R, Jaisankar TJ, Thappa DM, Kumari R, Chandrasekhar L, Malathi M, Parija SC, Habeebullah S (2013) Spectrum of vaginal discharge in a tertiary care setting. Trop Parasitol 3(2):135
Plato A, Hardison SE, Brown GD (2015) Pattern recognition receptors in antifungal immunity. In: Seminars in immunopathology, vol 37(2). Springer, Berlin, pp 97–106
Verstraelen H, Verhelst R, Nuytinck L, Roelens K, De Meester E, De Vos D, Van Thielen M, Rossau R, Delva W, De Backer E, Vaneechoutte M (2009) Gene polymorphisms of Toll-like and related recognition receptors in relation to the vaginal carriage of Gardnerella vaginalis and Atopobium vaginae. J Reprod Immunol 79(2):163–73
Cauci S, Guaschino S, De Aloysio D, Driussi S, De Santo D, Penacchioni P, Quadrifoglio F (2003) Interrelationships of interleukin-8 with interleukin-1β and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod 9(1):53–8
Zhang LJ, Gallo RL (2016) Antimicrobial peptides. Curr Biol 26(1):R14-9
Bahar AA, Ren D (2013) Antimicrobial peptides. Pharmaceuticals 6(12):1543–75
Diamond G, Beckloff N, Weinberg A, Kisich KO (2009) The roles of antimicrobial peptides in innate host defense. Curr Pharm Des 15(21):2377–92
Garcia-Gutierrez E, Mayer MJ, Cotter PD, Narbad A (2019) Gut microbiota as a source of novel antimicrobials. Gut microbes 10(1):1–21
Zhao L, Lu W (2014) Defensins in innate immunity. Curr Opin Hematol 21(1):37–42
Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT (2006) Human α-defensins block papillomavirus infection. Proc Natl Acad Sci 103(5):1516–21
Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T (2002) Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol 187(1):137–44
Sun L, Finnegan CM, Kish-Catalone T, Blumenthal R, Garzino-Demo P, La Terra Maggiore GM, Berrone S, Kleinman C, Wu Z, Abdelwahab S, Lu W (2005) Human β-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol 79(22):14318–29
Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC (1998) Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol 152(5):1247
Brunner A, Medvecz M, Makra N, Sárdy M, Komka K, Gugolya M, Szabó D, Gajdács M, Ostorházi E (2021) Human beta defensin levels and vaginal microbiome composition in post-menopausal women diagnosed with lichen sclerosus. Sci Rep 11(1):1–1
Han JH, Kim MS, Lee MY, Kim TH, Lee MK, Kim HR, Myung SC (2010) Modulation of human β-defensin-2 expression by 17β-estradiol and progesterone in vaginal epithelial cells. Cytokine 49(2):209–14
Yasin B, Wang W, Pang M, Cheshenko N, Hong T, Waring AJ, Herold BC, Wagar EA, Lehrer RI (2004) θ defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J Virol 78(10):5147–56
Tang LJ, De Seta F, Odreman F, Venge P, Piva C, Guaschino S, Garcia RC (2007) Proteomic analysis of human cervical-vaginal fluids. J Proteome Res 6(7):2874–83
Baker EN, Baker HM, Kidd RD (2002) Lactoferrin and transferrin: functional variations on a common structural framework. Biochem Cell Biol 80(1):27–34
Singh PK, Parsek MR, Greenberg EP, Welsh MJ (2002) A component of innate immunity prevents bacterial biofilm development. Nature 417(6888):552–5
Håversen LA, Engberg I, Baltzer L, Dolphin G, Hanson LÅ, Mattsby-Baltzer I (2000) Human lactoferrin and peptides derived from a surface-exposed helical region reduce experimental Escherichia coli urinary tract infection in mice. Infect Immun 68(10):5816–23
Pino A, Giunta G, Randazzo CL, Caruso S, Caggia C, Cianci A (2017) Bacterial biota of women with bacterial vaginosis treated with lactoferrin: an open prospective randomized trial. Microb Ecol Health Dis 28(1):1357417
Rein MF, Shih LM, Miller JR, Guerrant RL (1996) Use of a lactoferrin assay in the differential diagnosis of female genital tract infections and implications for the pathophysiology of bacterial vaginosis. Sex Transm Dis 1:517–21
Deruaz M, Luster AD (2015) Chemokine-mediated immune responses in the female genital tract mucosa. Immunol Cell Biol 93(4):347–54
Srakaew N, Young CD, Sae-Wu A, Xu H, Quesnel KL, Di Brisco R, Kongmanas K, Fongmoon D, Hommalai G, Weerachatyanukul W, Hall SH (2014) Antimicrobial host defence peptide, LL-37, as a potential vaginal contraceptive. Hum Reprod 29(4):683–96
Frew L, Makieva S, McKinlay AT, McHugh BJ, Doust A, Norman JE, Davidson DJ, Stock SJ (2014) Human cathelicidin production by the cervix. PLoS ONE 9(8):e103434
Larrick JW, Hirata M, Zhong J, Wright SC (1995) Anti-microbial activity of human CAP18 peptides. Immunotechnology 1(1):65–72
Penberthy WT, Chari S, Cole AL, Cole AM (2011) Retrocyclins and their activity against HIV-1. Cell Mol Life Sci 68(13):2231–42
Cho Y, Turner JS, Dinh NN, Lehrer RI (1998) Activity of protegrins against yeast-phase Candida albicans. Infect Immun 66(6):2486–93
MacNeill C, Umstead TM, Phelps DS, Lin Z, Floros J, Shearer DA, Weisz J (2004) Surfactant protein A, an innate immune factor, is expressed in the vaginal mucosa and is present in vaginal lavage fluid. Immunology 111(1):91–9
Leth-Larsen R, Floridon C, Nielsen O, Holmskov U (2004) Surfactant protein D in the female genital tract. MHR Basic Sci Reprod Med 10(3):149–54
Wyatt KA, Filby CE, Davies-Tuck ML, Suke SG, Evans J, Gargett CE (2021) Menstrual fluid endometrial stem/progenitor cell and supernatant protein content: cyclical variation and indicative range. Hum Reprod 36(8):2215–29
Venkataraman N, Cole AL, Svoboda P, Pohl J, Cole AM (2005) Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol 175(11):7560–7
Abtin A, Eckhart L, Gläser R, Gmeiner R, Mildner M, Tschachler E (2010) The antimicrobial heterodimer S100A8/S100A9 (calprotectin) is upregulated by bacterial flagellin in human epidermal keratinocytes. J Investig Dermatol 130(10):2423–30
Nakashige TG, Zhang B, Krebs C, Nolan EM (2015) Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol 11(10):765–71
Pellis V, De Seta F, Crovella S, Bossi F, Bulla R, Guaschino S, Radillo O, Garred P, Tedesco F (2005) Mannose binding lectin and C3 act as recognition molecules for infectious agents in the vagina. Clin Exp Immunol 139(1):120–6
Zegels G, Van Raemdonck GA, Tjalma WA, Van Ostade XW (2010) Use of cervicovaginal fluid for the identification of biomarkers for pathologies of the female genital tract. Proteome Sci 8(1):1–23
Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV (2011) Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol 65(3):196–211
Yarbrough VL, Winkle S, Herbst-Kralovetz MM (2015) Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update 21(3):353–77
Hernández-González JC, Martínez-Tapia A, Lazcano-Hernández G, García-Pérez BE, Castrejón-Jiménez NS (2021) Bacteriocins from lactic acid bacteria. A powerful alternative as antimicrobials, probiotics, and immunomodulators in veterinary medicine. Animals 11(4):979
Donia MS, Fischbach MA (2015) Small molecules from the human microbiota. Science 349(6246):1254766
Caplice E, Fitzgerald GF (1999) Food fermentations: role of microorganisms in food production and preservation. Int J Food Microbiol 50(1–2):131–49
Goldin BR (1998) Health benefits of probiotics. Br J Nutr 80(S2):S203-7
Riaz QU, Masud T (2013) Recent trends and applications of encapsulating materials for probiotic stability. Crit Rev Food Sci Nutr 53(3):231–44
Ansari JM, Colasacco C, Emmanouil E, Kohlhepp S, Harriott O (2019) Strain-level diversity of commercial probiotic isolates of Bacillus, Lactobacillus, and Saccharomyces species illustrated by molecular identification and phenotypic profiling. PLoS ONE 14(3):e0213841
Tytgat HL, van Teijlingen NH, Sullan RM, Douillard FP, Rasinkangas P, Messing M, Reunanen J, Satokari R, Vanderleyden J, Dufrene YF, Geijtenbeek TB (2016) Probiotic gut microbiota isolate interacts with dendritic cells via glycosylated heterotrimeric pili. PLoS ONE 11(3):e0151824
Scillato M, Spitale A, Mongelli G, Privitera GF, Mangano K, Cianci A, Stefani S, Santagati M (2021) Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. MicrobiologyOpen 10(2):e1173
Anukam K, Osazuwa E, Ahonkhai I, Ngwu M, Osemene G, Bruce AW, Reid G (2006) Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect 8(6):1450–4
Antonio MA, Meyn LA, Murray PJ, Busse B, Hillier SL (2009) Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous Lactobacilli. J Infect Dis 199(10):1506–13
Bruce AW, Reid G (1988) Intravaginal instillation of lactobacilli for prevention of recurrent urinary tract infections. Can J Microbiol 34(3):339–43
Othman M, Alfirevic Z, Neilson JP (2007) Probiotics for preventing preterm labour. Cochrane Datab Systematic Rev 1:1
Vitali B, Cruciani F, Baldassarre ME, Capursi T, Spisni E, Valerii MC, Candela M, Turroni S, Brigidi P (2012) Dietary supplementation with probiotics during late pregnancy: outcome on vaginal microbiota and cytokine secretion. BMC Microbiol 12(1):1–4
Zuccotti G, Meneghin F, Aceti A, Barone G, Callegari ML, Di Mauro A, Fantini MP, Gori D, Indrio F, Maggio L, Morelli L (2015) Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy 70(11):1356–71
Baldassarre ME, Di Mauro A, Mastromarino P, Fanelli M, Martinelli D, Urbano F, Capobianco D, Laforgia N (2016) Administration of a multi-strain probiotic product to women in the perinatal period differentially affects the breast milk cytokine profile and may have beneficial effects on neonatal gastrointestinal functional symptoms. A randomized clinical trial. Nutrients 8(11):677
Gupta V, Nag D, Garg P (2017) Recurrent urinary tract infections in women: how promising is the use of probiotics? Indian J Med Microbiol 35(3):347–54
Wang Z, He Y, Zheng Y (2019) Probiotics for the treatment of bacterial vaginosis: a meta-analysis. Int J Environ Res Public Health 16(20):3859
Hemalatha R, Mastromarino P, Ramalaxmi BA, Balakrishna NV, Sesikeran B (2012) Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: a randomized, double-blind study. Eur J Clin Microbiol Infect Dis 31(11):3097–105
Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, Bruce AW (2003) Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol Med Microbiol 35(2):131–4
Mastromarino P, Macchia S, Meggiorini L, Trinchieri V, Mosca L, Perluigi M, Midulla C (2009) Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infect 15(1):67–74
Martinez RC, Franceschini SA, Patta MC, Quintana SM, Candido RC, Ferreira JC, De Martinis EC, Reid G (2009) Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett Appl Microbiol 48(3):269–74
Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, Stamm WE (2011) Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis 52(10):1212–7
Czaja CA, Stapleton AE, Yarova-Yarovaya Y, Stamm WE (2007) Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect Dis Obstet Gynecol 2007:1
Ngugi BM, Hemmerling A, Bukusi EA, Kikuvi G, Gikunju J, Shiboski S, Fredricks DN, Cohen CR (2011) Effects of BV-associated bacteria and sexual intercourse on vaginal colonization with the probiotic Lactobacillus crispatus CTV-05. Sex Transm Dis 38(11):1020
Pendharkar S, Brandsborg E, Hammarström L, Marcotte H, Larsson PG (2015) Vaginal colonisation by probiotic lactobacilli and clinical outcome in women conventionally treated for bacterial vaginosis and yeast infection. BMC Infect Dis 15(1):1–2
Tomusiak A, Strus M, Heczko PB, Adamski P, Stefański G, Mikołajczyk-Cichońska A, Suda-Szczurek M (2015) Efficacy and safety of a vaginal medicinal product containing three strains of probiotic bacteria: a multicenter, randomized, double-blind, and placebo-controlled trial. Drug Des Dev Ther 9:5345
Ou YC, Fu HC, Tseng CW, Wu CH, Tsai CC, Lin H (2019) The influence of probiotics on genital high-risk human papilloma virus clearance and quality of cervical smear: a randomized placebo-controlled trial. BMC Womens Health 19(1):1–7
Martín V, Cárdenas N, Ocaña S, Marín M, Arroyo R, Beltrán D, Badiola C, Fernández L, Rodríguez JM (2019) Rectal and vaginal eradication of Streptococcus agalactiae (GBS) in pregnant women by using Lactobacillus salivarius CECT 9145, a target-specific probiotic strain. Nutrients 11(4):810
Brown KL, Hancock RE (2006) Cationic host defense (antimicrobial) peptides. Curr Opin Immunol 18(1):24–30
Mahlapuu M, Björn C, Ekblom J (2020) Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit Rev Biotechnol 40(7):978–92
Russo R, Superti F, Karadja E, De Seta F (2019) Randomised clinical trial in women with recurrent vulvovaginal candidiasis: efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses 62(4):328–35
Russo R, Karadja E, De Seta F (2019) Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: a double blind, placebo controlled, randomised clinical trial. Beneficial Microbes 10(1):19–26
Tanphaichitr N, Srakaew N, Alonzi R, Kiattiburut W, Kongmanas K, Zhi R, Li W, Baker M, Wang G, Hickling D (2016) Potential use of antimicrobial peptides as vaginal spermicides/microbicides. Pharmaceuticals 9(1):13
Huan Y, Kong Q, Mou H, Yi H (2020) Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 2559:1
Reddy KV, Yedery RD, Aranha C (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24(6):536–47
Galdiero S, Falanga A, Tarallo R, Russo L, Galdiero E, Cantisani M, Morelli G, Galdiero M (2013) Peptide inhibitors against herpes simplex virus infections. J Pept Sci 19(3):148–58
Ballweber LM, Jaynes JE, Stamm WE, Lampe MF (2002) In vitro microbicidal activities of cecropin peptides D2A21 and D4E1 and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis. Antimicrobial Agents Chemother 46(1):34–41
Sambri V, Marangoni A, Giacani L, Gennaro R, Murgia R, Cevenini R, Cinco M (2002) Comparative in vitro activity of five cathelicidin-derived synthetic peptides against Leptospira, Borrelia and Treponema pallidum. J Antimicrob Chemother 50(6):895–902
Wachinger M, Kleinschmidt A, Winder D, von Pechmann N, Ludvigsen A, Neumann M, Holle R, Salmons B, Erfle V, Brack-Werner R (1998) Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J Gen Virol 79(4):731–40
Tamamura H, Kuroda M, Masuda M, Otaka A, Funakoshi S, Nakashima H, Yamamoto N, Waki M, Matsumoto A, Lancelin JM, Kohda D (1993) A comparative study of the solution structures of tachyplesin I and a novel anti-HIV synthetic peptide, T22 ([Tyr5, 12, Lys7]-polyphemusin II), determined by nuclear magnetic resonance. Biochim Biophysica Acta (BBA) Protein Struct Mol Enzymol 1163(2):209–16
Giacometti A, Cirioni O, Ghiselli R, Goffi L, Mocchegiani F, Riva A, Scalise G, Saba V (2000) Polycationic peptides as prophylactic agents against methicillin-susceptible or methicillin-resistant Staphylococcus epidermidis vascular graft infection. Antimicrob Agents Chemother 44(12):3306–9
Haukland HH, Ulvatne H, Sandvik K, Vorland LH (2001) The antimicrobial peptides lactoferricin B and magainin 2 cross over the bacterial cytoplasmic membrane and reside in the cytoplasm. FEBS Lett 508(3):389–93
Goraya J, Knoop FC, Conlon JM (1999) Ranatuerin 1T: an antimicrobial peptide isolated from the skin of the frog. Rana temporaria Peptides 20(2):159–63
Park N, Yamanaka K, Tran D, Chandrangsu P, Akers JC, de Leon JC, Morrissette NS, Selsted ME, Tan M (2009) The cell-penetrating peptide, Pep-1, has activity against intracellular chlamydial growth but not extracellular forms of Chlamydia trachomatis. J Antimicrob Chemother 63(1):115–23
Madanchi H, Shoushtari M, Kashani HH, Sardari S (2020) Antimicrobial peptides of the vaginal innate immunity and their role in the fight against sexually transmitted diseases. New Microbes New Infect 34:100627
Samot J, Rouabhia M (2021) Effect of dermaseptin S4 on C. albicans growth and EAP1 and HWP1 gene expression. Probiot Antimicrob Prot 13(1):287–98
Navon-Venezia S, Feder R, Gaidukov L, Carmeli Y, Mor A (2002) Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob Agents Chemother 46(3):689–94
Belaid A, Aouni M, Khelifa R, Trabelsi A, Jemmali M, Hani K (2002) In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J Med Virol 66(2):229–34
Hancock RE (1997) Peptide antibiotics. Lancet 349(9049):418–22
Lin PF, Samanta H, Bechtold CM, Deminie CA, Patick AK, Alam M, Riccardi K, Rose RE, White RJ, Colonno RJ (1996) Characterization of siamycin I, a human immunodeficiency virus fusion inhibitor. Antimicrob Agents Chemother 40(1):133–8
Algburi A, Zehm S, Netrebov V, Bren AB, Chistyakov V, Chikindas ML (2017) Subtilosin prevents biofilm formation by inhibiting bacterial quorum sensing. Probiot Antimicrob Proteins 9(1):81–90
Abbasi J (2019) Are probiotics money down the toilet? Or worse? JAMA 321(7):633–5
Marcotte H, Larsson PG, Andersen KK, Zuo F, Mikkelsen LS, Brandsborg E, Gray G, Laher F, Otwombe K (2019) An exploratory pilot study evaluating the supplementation of standard antibiotic therapy with probiotic lactobacilli in south African women with bacterial vaginosis. BMC Infect Dis 19(1):1–5
Larsson PG, Stray-Pedersen B, Ryttig KR, Larsen S (2008) Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Womens Health 8(1):1–8
Witt A, Kaufmann U, Bitschnau M, Tempfer C, Özbal A, Haytouglu E, Gregor H, Kiss H (2009) Monthly itraconazole versus classic homeopathy for the treatment of recurrent vulvovaginal candidiasis: a randomised trial. BJOG Int J Obstet Gynaecol 116(11):1499–505
Khalesi S, Bellissimo N, Vandelanotte C, Williams S, Stanley D, Irwin C (2019) A review of probiotic supplementation in healthy adults: Helpful or hype? Eur J Clin Nutr 73(1):24–37
Buggio L, Somigliana E, Borghi A, Vercellini P (2019) Probiotics and vaginal microecology: Fact or fancy? BMC Womens Health 19(1):1–6
Donders GG, Ravel J, Vitali B, Netea MG, Salumets A, Unemo M (2017) Role of molecular biology in diagnosis and characterization of vulvo-vaginitis in clinical practice. Gynecol Obstet Invest 82(6):607–16
Bafeta A, Koh M, Riveros C, Ravaud P (2018) Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med 169(4):240–7
van de Wijgert JH, Verwijs MC (2020) Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: a systematic review and recommendations for future trial designs. BJOG Int J Obstet Gynaecol 127(2):287–99
Suez J, Zmora N, Segal E, Elinav E (2019) The pros, cons, and many unknowns of probiotics. Nat Med 25(5):716–29
Ebenhan T, Gheysens O, Kruger HG, Zeevaart JR, Sathekge MM (2014) Antimicrobial peptides: their role as infection-selective tracers for molecular imaging. BioMed Res Int 2014:1
Zhang L, Falla TJ (2006) Antimicrobial peptides: therapeutic potential. Expert Opin Pharmacother 7(6):653–63
Habets MG, Brockhurst MA (2012) Therapeutic antimicrobial peptides may compromise natural immunity. Biol Let 8(3):416–8
Kang SJ, Park SJ, Mishig-Ochir T, Lee BJ (2014) Antimicrobial peptides: therapeutic potentials. Expert Rev Anti Infect Ther 12(12):1477–86
Acknowledgements
NA, the authors mentioned above are solely responsible for this manuscript.
Funding
No funding was obtained for this study, the review paper has been constructed without the need of any financial assistance from any funding agency.
Author information
Authors and Affiliations
Contributions
SD and BK are responsible for the conception of idea for this review paper. She drafted, constructed and critically revised this work. She approves and hold accountability for this manuscript and is willing to answer any queries about the manuscript. BK is responsible for hel** SD in drafting and thoroughly revising this manuscript. He helped in improving the scientific aspect of this manuscript and is also willing to answer any queries about the manuscript. Both authors have jointly scripted, revised and approved this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Financial interest—Author BK is a full time Senior Professor at Tezpur University, Central, Assam, India and receives salary from the institution. Author SD is a full time Ph.D. scholar at Tezpur University, Central, Assam, India working under Author BK, she was paid institutional fellowship by the institute from 2017 to 2021 of her PhD tenure. Non-financial interest—none.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, S., Konwar, B.K. Influence of connatural factors in sha** vaginal microflora and ensuring its health. Arch Gynecol Obstet 309, 871–886 (2024). https://doi.org/10.1007/s00404-023-07200-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07200-8