Abstract
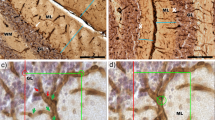
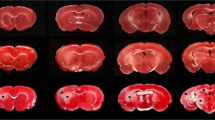
Pathological effects of moderate ischemia (oligemia, hypoperfusion) are relevant in relation to vascular factors in dementia. Chronic bilateral common carotid artery occlusion (BCCAO) in adult Wistar rats induces oligemia and leads to acute changes in gene expression, subacute changes in cortical astrocytes and prolonged changes in white matter tracts, while largely sparing neurons in the forebrain areas. Dilation and remodeling of the basilar artery ensures blood flow to the forebrain. The present study examined the hypoxia-sensitive Purkinje cells in the cerebellum after 6 months of BCCAO using conventional neuropathological analysis, immunohistochemistry and high-precision design-based stereologic methods. Purkinje cells in the vermis region revealed abnormally shaped nuclei. A stereologic analysis showed that the mean total number of Purkinje cells within the vermis was statistically significantly smaller in the BCCAO animals than in the control animals (d = 11.8%; P < 0.0001). BCCAO had no significant effect on the mean volumes of the molecular layer, granule cell layer and white matter in the vermis or the entire cerebellum. Remodeling of the basilar artery indicated that secondary vascular perturbations might be responsible for the effects of BCCAO on the cerebellar Purkinje cells.




Similar content being viewed by others
References
Andersen BB, Gundersen HJ, Pakkenberg B (2003) Aging of the human cerebellum: a stereological study. J Comp Neurol 466:356–365
Armstrong DM, Schild RF (1970) A quantitative study of the Purkinje cells in the cerebellum of the albino rat. J Comp Neurol 139:449–456
Balchen T, Diemer NH (1992) The AMPA antagonist NBQX protects against ischemia-induced loss of cerebellar Purkinje cells. Neuroreport 3:785–788
Barclay LL, Brady PA (1992) Cerebellar atrophy as a CT marker for mixed dementia. Biol Psychiatry 31:520–524
Bedi KS, Campbell LF, Mayhew TM (1992) A fractionator study of the effects of undernutrition during early life on rat Purkinje cell numbers (with a caveat on the use of nucleoli as counting units). J Anat 181:199–208
Bower AJ (1986) The number of Purkinje cells in the adult rat cerebellum. Neurosci Lett Suppl 24:S46
Bowler JV (2004) Vascular cognitive impairment. Stroke 35:386–388
Casserly I, Topol E (2004) Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet 363:1139–1146
Cavalieri B (1635) Geometria indivisibilibus continuorum. Typis Clementis Ferronij Bononiae. Reprinted 1966 as Geometria Degli Indivisibili, Unione Tipografico-Editrice, Torinese, Torino
Cavanagh JB, Holton JL, Nolan CC (1997) Selective damage to the cerebellar vermis in chronic alcoholism: a contribution from neurotoxicology to an old problem of selective vulnerability. Neuropathol Appl Neurobiol 23:355–363
Celio MR (1990) Calbindin D-28 k and parvalbumin in the rat nervous system. Neuroscience 35:375–475
Cervos-Navarro J Diemer NH (1991) Selective vulnerability in brain hypoxia. Crit Rev Neurobiol 6:149–182
Choki JI, Yamaguchi T, Takeya Y, Morotomi Y, Omae T (1977) Effect of carotid artery ligation on regional cerebral blood flow in normotensive and spontaneously hypertensive rats. Stroke 8:374–379
Choy MK, Ganesan V, Thomas DL, Thornton JS, Proctor E, King MD, van der Weerd L, Gadian DG, Lythdoe MF (2006) The chronic vascular and hemodynamic response after permanent bilateral common carotid occlusion in newborn and adult rats. J Cereb Blood Flow Metab 26:1066–1075
De Butte M, Fortin T, Pappas BA (2002) Pinealectomy: behavioral and neuropathological consequences in a chronic cerebral hypoperfusion model. Neurobiol Aging 23:309–317
De la Torre JC (2004) Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 3:184–190
DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ (2006) The developmental neurobiology of autism spectrum disorder. J Neurosci 26:6897–906
Farkas E, Luiten PGM (2001) Cerebral microvascular pathology in aging and Alzheimer’s disase. Prog Neurobiol 64:575–611
Fujii K, Heistad DD, Faraci FM (1991) Flow-mediated dilatation of the basilar artery in vivo. Circ Res 69:697–705
Gold L, Lauritzen M (2002) Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc Natl Acad Sci USA 99:7699–7704
Harvey RJ, Napper RMA (1988) Quantitative study of granule and Purkinje cells in the cerebellar cortex of the rat. J Comp Neurol 274:151–157
Hawkes R, Colonnier M, Leclerc N (1985) Monoclonal antibodies reveal sagittal banding in the rodent cerebellar cortex. Brain Res 333:359–365
He Z, Ibayashi S, Sugimori H, Fujii K, Sadoshima S, Fujishima M (1997) Age-related ischemia in the brain following bilateral carotid artery occlusion–collateral blood flow and brain metabolism. Neurochem Res 22:37–42
Hillman DE, Chen S (1981) Vulnerability of cerebellar development in malnutrition-I Quantitation of layer volume and neuron numbers. Neuroscience 6:1249–1262
Iadecola C (2004) Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5:347–360
Jellinger KA (2005) Understanding the pathology of vascular cognitive impairment. J Neurol Sci 229:57–63
Kalaria RN (2000) The role of cerebral ischemia in Alzheimer’s disease. Neurobiol Aging 21:321–330
Larsen JO, Skalicky M, Viidik A (2000) Does long-term physical exercise counteract age-related Purkinje cell loss? A stereological study of rat cerebellum. J Comp Neurol 428:213–222
Larsen AA, Stoltenberg M, West MJ, Danscher G (2005) Influence of bismuth on the number of neurons in cerebellum and hippocampus of normal and hypoxia-exposed mouse brain: a stereological study. J Appl Toxicol 25:383–365
Luft AR, Skalej M, Schulz JB, Welte D, Kolb R, Buerk K, Klockgether T, Voigt K (1999) Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumentry. Cerebral Cortex 9:712–721
Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, Cao Y, Berkenstam A, Poellinger L (2001) Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414:550–554
Mielke R, Herholz K, Grond M, Kessler J, Heiss WD (1992) Severity of vascular dementia is related to volume of metabolically impaired tissue. Arch Neurol 49:909–913
Mies G, Ishimaru S, **e Y, Seo K, Hossmann KA (1991) Ischemic thresholds of cerebral protein synthesis and energy state following middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab 11:753–761
Nakanishi S, Maeda N, Mikoshiba K (1991) Immunohistochemical localization of an inositol 145-triphosphate receptor P400 in neural tissue: studies in develo** and adult mouse brain. J Neurosci 11:2075–2086
O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST (2003) Vascular cognitive impairment. Lancet Neurol 2:89–98
Ohta H, Nishikawa H, Kimura H, Anayama H, Miyamoto M (1997) Chronic cerebral hypoperfusion by permanent internal carotid artery ligation produces learning impairment without brain damage in rats. Neuroscience 79:1039–1050
Oldendorf WH (1989) Trophic changes in the arteries at the base of the rat brain in response to bilateral common carotid ligation. J Neuropathol Exp Neurol 48:534–547
Ouchi Y, Tsukada H, Kakiuchi T, Nishiyama S, Futatsubashi M (1998) Changes in cerebral blood flow and postsynaptic muscarinic cholinergic activity in rats with bilateral carotid artery ligation. J Nucl Med 39:198–202
Otori T, Katsumata T, Muramatsu H, Kashiwagi F, Katayama Y, Terashi A (2003) Long-term measurement of cerebral blood flow and metabolism in a rat chronic hypoperfusion model. Clin Exp Pharmacol Physiol 30:266–272
Pae EK, Chien P, Harper RM (2005) Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett 375:123–128
Palladini G, Conforti A, Medolago-Albani L (1976) Ultrastructural hypoxic changes in Ammon’s horn and Purkinje cells. Brain Res 103:45–56
Pantel J, Schroder J, Essig M, Jauss M, Schneider G, Eysenbach K, von Kummer R, Baudendistel K, Schad LR, Knopp MV (1998) In vivo quantification of brain volumes in subcortical vascular dementia and Alzheimer’s disease. An MRI-based study. Dement Geriatr Cogn Disord 9:309–316
Paxinos G (1995) The rat nervous system. Academic, San Diego
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic, San Diego
Roman GC, Royall DR (2004) A diagnostic dilemma: is “Alzheimer’s dementia” Alzheimer’s disease, vascular dementia, or both? Lancet Neurol 3:141
Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MMB (2005) Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol 57:789–794
Rutten BP, Wirths O, Van de Berg WD, Lichtenthaler SF, Vehoff J, Steinbusch HW, Korr H, Beyreuther K, Multhaup G, Bayer TA, Schmitz C (2003) No alterations of hippocampal neuronal number and synaptic bouton number in a transgenic mouse model expressing the beta-cleaved C-terminal APP fragment. Neurobiol Dis 12:110–120
Schaper W, Buschmann I (1999) Arteriogenesis, the good and bad of it. Cardiovasc Res 43:835–837
Schmahmann JD (2004) Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 16:367–78
Schmidt-Kastner R, Truettner J, Zhao W, Saul I, Busto R, Ginsberg MD (2001) Transient changes of brain-derived neurotrophic factor (BDNF) mRNA expression in hippocampus during moderate ischemia induced by bilateral common carotid artery occlusions in the rat. Mol Brain Res 92:157–166
Schmidt-Kastner R, Aguirre-Chen C, Saul I, Yick L, Hamasaki D, Busto R, Ginsberg MD (2005) Astrocytes react to oligemia in the forebrain induced by chronic bilateral common carotid artery occlusion in rats. Brain Res 1052:28–39
Schmitz C (1998) Variation of fractionator estimates and its prediction. Anat Embryol 198:371–397
Schmitz C, Hof PR (2000) Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat 20:93–114
Schmitz C, Hof PR (2005) Design-based stereology in neuroscience. Neuroscience 130:813–831
Schmitz C, Born M, Dolezel P, Rutten BPF, de Saint-Georges L, Hof PR, Korr H (2005) Prenatal protracted irradiation at very low dose rate induces severe neuronal loss in rat hippocampus and cerebellum. Neuroscience 130:935–948
Scremin OU (1995) Cerebral vascular system. In: Paxinos G (ed) The rat nervous system 2nd edn. Academic, San Diego, pp 3–35
Thal DR, Ghebremedhin E, Orantes M, Wiestler OD (2003) Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 62:1287–1301
Torvik A, Torp S, Lindboe CF (1986) Atrophy of the cerebellar vermis in ageing. A morphometric and histologic study. J Neurol Sci 76:283–294
Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O’Hearn E, Molliver ME, Aicher SA (2002) Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4 and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol 89:331–359
West MJ, Slomianka L, Gundersen HJG (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497
Xu J, Liu Z, Ornitz DM (2000) Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development 127:1833–1843
Yamamoto H, Schmidt-Kastner R, Hamasaki DI, Yamamoto H, Parel JM (2006) Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp Eye Res 82:767–779
Yu MC, Bakay L, Lee JC (1972) Ultrastructure of the central nervous system after prolonged hypoxia. I Neuronal alterations. Acta Neuropathol 22:222–234
Acknowledgments
The authors wish to thank Helga Helten and Susanne Echterhagen for their excellent technical assistance. This study was supported by the US Public Health Service grant NS 05820-S361. C.S. was supported by the US National Alliance for Autism Research (NAAR) and the Internationale Stichting Alzheimer Onderzoek (ISAO, The Netherlands).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kántor, O., Schmitz, C., Feiser, J. et al. Moderate loss of cerebellar Purkinje cells after chronic bilateral common carotid artery occlusion in rats. Acta Neuropathol 113, 549–558 (2007). https://doi.org/10.1007/s00401-007-0204-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-007-0204-y