Abstract
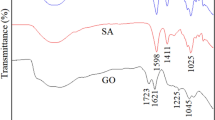
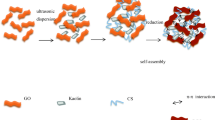
Sodium alginate gels modified by polyvinyl alcohol and graphene oxide (GO/PVA/SA) were prepared, and were characterized by Fourier-transform infrared spectroscopy, Raman spectrum, and X–ray diffraction analysis. The morphologies of the gels were observed with scanning electron microscope. It was found that the adsorption of polyvinyl alcohol/sodium alginate gels (PVA/SA) could be modified due to the incorporation of graphene oxide. The adsorption data showed that the adsorption capacity of GO/PVA/SA gel spheres was higher than that of PVA/SA gel spheres and was optimal when the graphene oxide content reached 4 wt% in composite gel spheres. The adsorption kinetics had a better agreement with the pseudo-second-order equation than the pseudo-first-order equation, and Langmuir model was more suitable for explaining the current adsorption process of gels for dye. The desorption behavior of dye showed that the gel spheres could keep a certain adsorption capacity after 4 cycles and the desorption effects of gel with the 4 wt% graphene oxide reached the optimal level.
Graphical abstract
















Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Shalla AH, Bhat MA, Yaseen Z (2018) Hydrogels for removal of recalcitrant organic dyes: a conceptual overview. J Environ Chem Eng 6(5):5938–5949
Wang AQ, Wu SS, Zhang YN, Miao LJ, Zhang YJ, Wu AG (2020) Preparation of modified sodium alginate aerogel and its application in removing lead and cadmium ions in wastewater. Int J Bio Macromol 157:687–694
Fernando IPS, Lee W, Han EJ, Ahn G (2020) Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem Eng J 391:123823
Dewangan T, Tiwari A, Bajpai AK (2009) Removal of cobalt ions from aqueous solution by adsorption onto cross-linked calcium alginate beads. J Disper Scie Technol 30(1):56–60
Thakur S, Sharma B, Verma A, Chaudhary J, Tamulevicius S, Thakur VK (2018) Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J Clean Prod 198:143–159
Bukhari AAH, Elsayed NH, Monier M (2021) Development and characterization of photo-responsive cinnamoly modified alginate. J Clean Prod 260:117771
Flores-Cespedes F, Villafranca-Sanchez M, Fernandez-Perez M (2020) Alginate-based hydrogels modified with olive pomace and lignin to removal organic pollutants from aqueous solutions. Int J Bio Macromol 153:883–891
Tang SX, Yang JY, Lin LZ, Peng KL, Chen Y, ** SH, Yao WS (2020) Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem Eng J 393:124728
Majhi D, Patra BN (2020) Polyaniline and sodium alginate nanocomposite: a pH-responsive adsorbent for the removal of organic dyes from water. RSC Adv 10(71):13904–43914
Ma J, Jiang Z, Cao JL, Yu F (2020) Enhanced adsorption for the removal of antibiotics by carbon nanotubes/graphene oxide/sodium alginate triple-network nanocomposite hydrogels in aqueous solutions. Chemosphere 242:125188
Chang SQ, Wang BX, Liu YZ, Li Z, Hu XD, Zhang XH, Zhang HQ (2020) Radiation-assistant preparation of highly conductive, transparent and self-healing hydrogels with triple-network structure. Polymer 188:122156
Jia YG, ** JH, Liu S, Ren L, Luo JT, Zhu XX (2018) Self-healing hydrogels of low molecular weight poly(vinyl alcohol) assembled by host-guest recognition. Biomacromol 19(2):626–632
Seera SDK, Kundu D, Banerjee T (2020) Physical and chemical crosslinked microcrystalline cellulose-polyvinyl alcohol hydrogel: freeze–thaw mediated synthesis, characterization and in vitro delivery of 5-fluorouracil. Cellulose 27(11):6521–6535
Yue YY, Han JQ, Han GP, French AD, Qi YD, Wu QL (2016) Cellulose nanofibers reinforced sodium alginate-polyvinyl alcohol hydrogels: core-shell structure formation and property characterization. Carbohyd Polym 147:155–164
**ang YJ, Xu ZY, Wei YY, Zhou YY, Yang X, Yang Y, Yang J, Zhang JC, Luo L, Zhou Z (2019) Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J Environ Manage 237:128–138
Gusain R, Kumar N, Ray SS (2020) Recent advances in carbon nanomaterial-based adsorbents for water purification. Coordin Chem Rev 405:213111. https://doi.org/10.1016/j.ccr.2019.213111
Kumar N, Salehiyan R, Chauke V, Botlhoko OJ, Setshedi K, Scriba M, Masukume M, Ray SS (2021) Top-down synthesis of graphene: a comprehensive review. FlatChem 27:100224. https://doi.org/10.1016/j.fatc.2021.100224
Maswanganyi S, Gusain R, Kumar N, Fosso-Kankeu E, Waanders FB, Ray SS (2021) Bismuth molybdate nanoplates supported on reduced graphene oxide: an effective nanocomposite for the removal of naphthalene via adsorption−photodegradation. ACS Omega 6(26):16783–16794. https://doi.org/10.1021/acsomega.1c01296
Yao WH, Yu F, Ma J (2018) Preparation of alginate composite gel and its application in water treatment. Prog Chem 30(11):1722–1733
**ao W, Jiang XP, Liu X, Zhou WM, Garba ZN, Lawan I, Wang LW, Yuan ZH (2021) Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J Clean Prod 284:124773
Quesada HB, de Araújo TP, Vareschini DT et al (2020) Chitosan, alginate and other macromolecules as activated carbon immobilizing agents: a review on composite adsorbents for the removal of water contaminants. Int J Bio Macromol 164:2535–2549
Dikin DA, Stankovich S, Zimney EJ, Piner RD, Dommett GHD, Evmenenko G, Nguyen ST, Ruoff RS (2007) Preparation and characterization of graphene oxide paper. Nature 448(7152):457–460
Yi XF, Sun FL, Han ZH, Han FH, He JR, Ou MR, Gu JJ, Xu XP (2018) Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu (II) and U (VI) removal. Ecotox Environ Safe 158:309–318
Yu YR, Zhang G, Lin Y (2019) Preparation and adsorption mechanism of polyvinyl alcohol/graphene oxide-sodium alginate nanocomposite hydrogel with high Pb (II) adsorption capacity. J Appl Polym Sci 136(14):47318
Liu CY, Liu HY, **ong TH, Xu AR, Pan BL, Tang KY (2018) Graphene oxide reinforced alginate/PVA double network hydrogels for efficient dye removal. Polym 10(8):835
Ma YX, Li X, Shao WJ, Kou YL, Yang HP, Zhang DJ (2020) Fabrication of 3D porous polyvinyl alcohol/sodium alginate/graphene oxide spherical composites for the adsorption of methylene blue. J Nanosci Nanotechno 20(4):2205–2213
Lin DC, Shi M, Wei XX, Liu BX, Cao JF, Peng CS (2019) Development of an innovative capsule with three-dimension honeycomb architecture via one-step titration-gel method for the removal of methylene blue. Int J Bio Macromol 128:911–922
Khalid I, Ahmad M, Minhas MU, Barkat K (2018) Preparation and characterization of alginate-PVA-based semi-IPN: controlled release pH-responsive composites. Polym Bull 75(3):1075–1099
Eldin MSM, Soliman EA, Elzatahry AAF, Elaassar MR, Eweida BY, Elkady MF, Rahman AMA, Yossef ME (2019) Carboxylated alginate hydrogel beads for methylene blue removal: formulation, kinetic and isothermal studies. Desalin Water Treat 168:308–323
Funding
The authors gratefully acknowledge the National Natural Science Foundation of China (11872001), Anhui Science Fund for Distinguished Young Scholars (1808085J30) and the Doctoral Research Funds of Anhui University of Science and Technology (ZY016) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, X., Zhang, J., Feng, H. et al. The influence of graphene oxide content on adsorption of PVA/SA composite gel spheres. Colloid Polym Sci 301, 93–105 (2023). https://doi.org/10.1007/s00396-022-05046-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-022-05046-1