Abstract
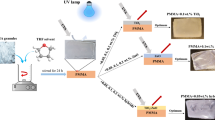
The oppositely charged titanium dioxide (TiO2) nanoparticles were deposited on the surface of poly(ethylene terephthalate) (PET) substrates by a layer-by-layer (LBL) assembly method. The PET films were pretreated by concentrated alkaline solution so as to introduce functional groups to attract TiO2 nanoparticles onto the surface. The oppositely charged TiO2 nanoparticles in the suspension can be controlled by adjusting the pH value. The multilayer buildup was monitored by UV-vis spectroscopy, which showed a linear increase of the film absorbance with the number of adsorbed TiO2 layers. Moreover, the morphology, structural, and chemical composition of the multilayer nanocomposite films were analyzed by scanning electron microscopy-energy dispersive X-ray analysis (SEM-EDX), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). The results confirmed the presence and even distribution of TiO2 nanoparticles on the PET surfaces. The UV protective properties, thermal stability, and mechanical properties of the nanocomposite films were investigated. The values of ultraviolet protection factor (UPF) showed that the multilayer nanocomposite films exhibited high UV protective property. The TGA curves showed that TiO2 nanoparticles enhanced the thermal stability of nanocomposite films. Additionally, the nanocomposite films showed a lower tensile strength but a higher elongation than that of PET film.










Similar content being viewed by others
References
Carneado S, Hernández-Nataren E, López-Sánchez JF (2015) Migration of antimony from polyethylene terephthalate used in mineral water bottles. Food Chem 166:544–550
Avella M, Bonadies E, Martuscelli E (2001) European current standardization for plastic packaging recoverable through composting and biodegradation. Polym Test 5:517–521
Nakaya M, Uedono A, Hotta A (2015) Recent progress in gas barrier thin film coatings on PET bottles in food and beverage applications. Coatings 4:987–1001
Wojciechowski K, Zukowska GZ, Korczagin I, Malanowski P (2015) Effect of TiO2 on UV stability of polymeric binder films used in waterborne facade paints. Prog Org Coat 85:123–130
Chen KS, Liu PY, Hung TS, Liao SC, Chen SC, Lin HR, Chen WY, Feng CK (2009) Preparation of titanium oxide-containing organic film by dip** Ti (OR)4 and cold plasma oxidizing on PET. Appl Surf Sci 256:1101–1105
Sawai Y, Nishimoto S, Kameshima Y (2013) Photoinduced underwater superoleophobicity of TiO2 thin films. Langmuir 23:6784–6789
EL-Mekkawi DM, Nady N, Abdelwahab NA (2016) Flexible bench-scale recirculating flow CPC photoreactor for solar photocatalytic degradation of methylene blue using removable TiO2 immobilized on PET sheets. Int J Photoenergy 2016:1–9
Sun ZG, Li XS, Zhu X (2014) Facile and fast deposition of amorphous TiO2 film under atmospheric pressure and at room temperature, and its high photocatalytic activity under UV-C light. Chem Vapor Depos 20:8–13
Artoshina OV, Milovich FO, Rossouw A (2016) Structure and phase composition of thin TiO2 films grown on the surface of metallized track-etched polyethylene terephthalate membranes by reactive magnetron sputtering. Inorg Mater 52:945–954
Peng XY, Ding EY, Xue F (2012) In situ synthesis of TiO2/polyethylene terephthalate hybrid nanocomposites at low temperature. Appl Surf Sci 258:6564–6570
Pandiyaraj KN, Deshmukh RR, Mahendiran R (2014) Influence of operating parameters on surface properties of RF glow discharge oxygen plasma treated TiO2/PET film for biomedical application. Mater Sci Eng C 36:309–319
** B, Verma LK, Li J (2012) TiO2 thin films prepared via adsorptive self-assembly for self-cleaning applications. ACS Appl Mater Interfaces 4:1093–1102
Decher G (1997) Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science 277:1232–1237
Hua F, Shi J, Lvov Y (2002) Patterning of layer-by-layer self-assembled multiple types of nanoparticle thin films by lithographic technique. Nano Lett 2:1219–1222
Li H, Pang S, Wu S (2011) Layer-by-layer assembly and UV photoreduction of graphene-polyoxometalate composite films for electronics. J Am Chem Soc 24:9423–9429
Su PG, Shieh HC (2014) Flexible NO2 sensors fabricated by layer-by-layer covalent anchoring and in situ reduction of graphene oxide. Sensor Actuat B Chem 190:865–872
Huang YL, Baji A, Tien HW (2012) Self-assembly of silver-graphene hybrid on electrospun polyurethane nanofibers as flexible transparent conductive thin films. Carbon 50:3473–3481
Li F, Biagioni P, Finazzi M, Tavazzi S, Piergiovanni L (2013) Tunable green oxygen barrier through layer-by-layer self-assembly of chitosan and cellulose nanocrystals. Carbohydr Polym 92:2128–2134
Yuan S, Mu J, Mao R (2014) All-nanoparticle self-assembly ZnO/TiO2 heterojunction thin films with remarkably enhanced photoelectrochemical activity. ACS Appl Mater Interfaces 6:5719–5725
Liu Y, He T, Gao C (2005) Surface modification of poly(ethylene terephthalate) via hydrolysis and layer-by-layer assembly of chitosan and chondroitin sulfate to construct cytocompatible layer for human endothelial cells. Colloids Surf B Biointerfaces 2:117–126
Kay A, Grätzel M (2002) Dye-sensitized core-shell nanocrystals: improved efficiency of mesoporous tin oxide electrodes coated with a thin layer of an insulating oxide. Chem Mater 7:2930–2935
Matsuno R, Otsuka H, Takahara A (2006) Polystyrene-grafted titanium oxide nanoparticles prepared through surface-initiated nitroxide-mediated radical polymerization and their application to polymer hybrid thin films. Soft Matter 5:415–421
Myroshnychenko V, Rodríguez-Fernández J, Pastoriza-Santos I (2008) Modelling the optical response of gold nanoparticles. Chem Soc Rev 9:1792–1805
Guo WZ, Lu H, Li XK (2016) Tungsten-promoted titania as solid acid for catalytic hydrolysis of waste bottle PET in supercritical CO2. RSC Adv 49:43171–43184
Osękowska M, Karuga-Kuźniewska E, Wojcieszak D (2015) Influence of nanocrystalline structure and surface properties of TiO2 thin films on the viability of L929 cells. Pol J Chem Technol 3:33–39
Sammon C, Yarwood J, Everall N (2000) An FT-IR study of the effect of hydrolytic degradation on the structure of thin PET films. Polym Degrad Stabil 67:149–158
Acknowledgements
This research was supported by the National Natural Science Foundation of China (51503164). Also, this research was supported by the Scientific Research Project of Hubei Provincial Department of Education (Q20171611) and the Wuhan Textiles University Research Fund (142078003, ZDSYS201712).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cheng, D., Cai, G., Wu, J. et al. UV protective PET nanocomposites by a layer-by-layer deposition of TiO2 nanoparticles. Colloid Polym Sci 295, 2163–2172 (2017). https://doi.org/10.1007/s00396-017-4178-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4178-6