Abstract
Purpose
The present study aimed at evaluating possible synergistic effects between two risk factors for cognitive decline and neurodegenerative disorders, i.e. iron overload and exposure to a hypercaloric/hyperlipidic diet, on cognition, insulin resistance, and hippocampal GLUT1, GLUT3, Insr mRNA expression, and AKT phosporylation.
Methods
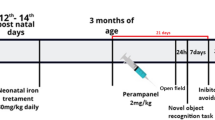
Male Wistar rats were treated with iron (30 mg/kg carbonyl iron) or vehicle (5% sorbitol in water) from 12 to 14th post-natal days. Iron-treated rats received a standard laboratory diet or a high fat diet from weaning to adulthood (9 months of age). Recognition and emotional memory, peripheral blood glucose and insulin levels were evaluated. Glucose transporters (GLUT 1 and GLUT3) and insulin signaling were analyzed in the hippocampus of rats.
Results
Both iron overload and exposure to a high fat diet induced memory deficits. Remarkably, the association of iron with the high fat diet induced more severe cognitive deficits. Iron overload in the neonatal period induced higher insulin levels associated with significantly higher HOMA-IR, an index of insulin resistance. Long-term exposure to a high fat diet resulted in higher fasting glucose levels. Iron treatment induced changes in Insr and GLUT1 expression in the hippocampus. At the level of intracellular signaling, both iron treatment and the high fat diet decreased AKT phosphorylation.
Conclusion
The combination of iron overload with exposure to a high fat diet only led to synergistic deleterious effect on emotional memory, while the effects induced by iron and by the high fat diet on AKT phosphorylation were comparable. These findings indicate that there is, at least to some extent, an additive effect of iron combined with the diet. Further studies investigating the mechanisms associated to deleterious effects on cognition and susceptibility for the development of age-associated neurodegenerative disorders are warranted.





Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Ashraf A, Clark M, So PW (2018) The aging of iron man. Front Aging Neurosci 10:65. https://doi.org/10.3389/fnagi.2018.00065
Kupershmidt L, Youdim MBH (2023) The neuroprotective activities of the novel multi-target iron-chelators in models of Alzheimer’s disease, amyotrophic lateral sclerosis and aging. Cells 12:763. https://doi.org/10.3390/cells12050763
Gao G, You L, Zhang J, Chang Y-Z, Yu P (2023) Brain iron metabolism, redox balance and neurological diseases. Antioxidants (Basel) 12:1289. https://doi.org/10.3390/antiox12061289
Holmes-Hampton GP, Chakrabarti M, Cockrell AL et al (2012) Changing iron content of the mouse brain during development. Metallomics 4:761–770. https://doi.org/10.1039/c2mt20086d
Miwa CP, de Lima MN, Scalco F et al (2011) Neonatal iron treatment increases apoptotic markers in hippocampal and cortical areas of adult rats. Neurotox Res 19:527–535. https://doi.org/10.1007/s12640-010-9181-3
Schröder N, Figueiredo LS, de Lima MN (2013) Role of brain iron accumulation in cognitive dysfunction: evidence from animal models and human studies. J Alzheimers Dis 34:797–812. https://doi.org/10.3233/JAD-121996
da Silva VK, de Freitas BS, da Silva DA et al (2014) Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection. Mol Neurobiol 49:222–233. https://doi.org/10.1007/s12035-013-8514-7
Figueiredo LS, de Freitas BS, Garcia VA et al (2016) Iron loading selectively increases hippocampal levels of ubiquitinated proteins and impairs hippocampus-dependent memory. Mol Neurobiol 53:6228–6239. https://doi.org/10.1007/s12035-015-9514-6
Alcalde LA, de Freitas BS, Machado GDB et al (2018) Iron chelator deferiprone rescues memory deficits, hippocampal BDNF levels and antioxidant defenses in an experimental model of memory impairment. Biometals 31:927–940. https://doi.org/10.1007/s10534-018-0135-1
da Silva VK, de Freitas BS, Garcia RCL et al (2018) Antiapoptotic effects of cannabidiol in an experimental model of cognitive decline induced by brain iron overload. Transl Psychiatry 8:176. https://doi.org/10.1038/s41398-018-0232-5
da Silva VK, de Freitas BS, Dornelles VC et al (2018) Novel insights into mitochondrial molecular targets of iron-induced neurodegeneration: Reversal by cannabidiol. Brain Res Bull 139:1–8. https://doi.org/10.1016/j.brainresbull.2018.01.014
Hare DJ, Arora M, Jenkins NL et al (2015) Is early-life iron exposure critical in neurodegeneration? Nat Rev Neurol 11:536–544. https://doi.org/10.1038/nrneurol.2015.100
Blasco G, Puig J, Daunis IEJ et al (2014) Brain iron overload, insulin resistance, and cognitive performance in obese subjects: a preliminary MRI case-control study. Diabetes Care 37:3076–3083. https://doi.org/10.2337/dc14-0664
Daugherty AM, Haacke EM, Raz N (2015) Striatal iron content predicts its shrinkage and changes in verbal working memory after two years in healthy adults. J Neurosci 35:6731–6743. https://doi.org/10.1523/JNEUROSCI.4717-14.2015
Traill WB, Mazzocchi M, Shankar B, Hallam D (2014) Importance of government policies and other influences in transforming global diets. Nutr Rev 72:591–604. https://doi.org/10.1111/nure.12134
Silva MVF, Loures CMG, Alves LCV et al (2019) Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci 26:33. https://doi.org/10.1186/s12929-019-0524-y
Pugazhenthi S, Qin L, Reddy PH (2017) Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis 1863:1037–1045. https://doi.org/10.1016/j.bbadis.2016.04.017
Morris JK, Bomhoff GL, Gorres BK et al (2011) Insulin resistance impairs nigrostriatal dopamine function. Exp Neurol 231:171–180. https://doi.org/10.1016/j.expneurol.2011.06.005
**ao Y, Gong X, Deng R et al (2022) Iron chelation remits memory deficits caused by the high-fat diet in a mouse model of Alzheimer’s disease. J Alzheimers Dis 86:1959–1971. https://doi.org/10.3233/JAD-215705
Petrov D, Pedros I, Artiach G et al (2015) High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiencies contribute to Alzheimer disease pathology in rodents. Biochim Biophys Acta 1852:1687–1699. https://doi.org/10.1016/j.bbadis.2015.05.004
Bracko O, Vinarcsik LK, Cruz Hernandez JC et al (2020) High fat diet worsens Alzheimer’s disease-related behavioral abnormalities and neuropathology in APP/PS1 mice, but not by synergistically decreasing cerebral blood flow. Sci Rep 10:9884. https://doi.org/10.1038/s41598-020-65908-y
Koepsell H (2020) Glucose transporters in brain in health and disease. Pflugers Arch 472:1299–1343. https://doi.org/10.1007/s00424-020-02441-x
Cholerton B, Baker LD, Craft S (2013) Insulin, cognition, and dementia. Eur J Pharmacol 719:170–179. https://doi.org/10.1016/j.ejphar.2013.08.008
Ahmed F, Ansari JA, Ansari ZE et al (2014) A molecular bridge: connecting type 2 diabetes and Alzheimer’s disease. CNS Neurol Disord Drug Targets 13:312–321. https://doi.org/10.2174/18715273113126660133
Percie du Sert N, Hurst V, Ahluwalia A et al (2020) The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br J Pharmacol 177:3617–3624. https://doi.org/10.1111/bph.15193
Pan Z, Lu XF, Shao C et al (2011) The effects of sevoflurane anesthesia on rat hippocampus: a genomic expression analysis. Brain Res 1381:124–133. https://doi.org/10.1016/j.brainres.2011.01.020
Zhang S, Hu X, Guan W et al (2017) Isoflurane anesthesia promotes cognitive impairment by inducing expression of beta-amyloid protein-related factors in the hippocampus of aged rats. PLoS ONE 12:e0175654. https://doi.org/10.1371/journal.pone.0175654
de Lima MN, Polydoro M, Laranja DC et al (2005) Recognition memory impairment and brain oxidative stress induced by postnatal iron administration. Eur J Neurosci 21:2521–2528. https://doi.org/10.1111/j.1460-9568.2005.04083.x
Molz P, de Freitas BS, Uberti VH et al (2021) Effects of lipoic acid supplementation on age- and iron-induced memory impairment, mitochondrial DNA damage and antioxidant responses. Eur J Nutr 60:3679–3690. https://doi.org/10.1007/s00394-021-02541-z
Schröder N, Fredriksson A, Vianna MR, Roesler R, Izquierdo I, Archer T (2001) Memory deficits in adult rats following postnatal iron administration. Behav Brain Res 124:77–85. https://doi.org/10.1016/s0166-4328(01)00236-4
Yoshimura TM, Sabino CP, Ribeiro MS (2016) Photobiomodulation reduces abdominal adipose tissue inflammatory infiltrate of diet-induced obese and hyperglycemic mice. J Biophotonics 9:1255–1262. https://doi.org/10.1002/jbio.201600088
Izquierdo I, Furini CR, Myskiw JC (2016) Fear memory. Physiol Rev 96:695–750. https://doi.org/10.1152/physrev.00018.2015
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Yun S, Wu Y, Niu R, Feng C, Wang J (2019) Effects of lead exposure on brain glucose metabolism and insulin signaling pathway in the hippocampus of rats. Toxicol Lett 310:23–30. https://doi.org/10.1016/j.toxlet.2019.04.011
Bonefeld BE, Elfving B, Wegener G (2008) Reference genes for normalization: a study of rat brain tissue. Synapse 62:302–309. https://doi.org/10.1002/syn.20496
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419. https://doi.org/10.1007/BF00280883
Nianogo RA, Rosenwohl-Mack A, Yaffe K, Carrasco A, Hoffmann CM, Barnes DE (2022) Risk factors associated with Alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol 79:584–591. https://doi.org/10.1001/jamaneurol.2022.0976
Strachan MW, Reynolds RM, Marioni RE, Price JF (2011) Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol 7:108–114. https://doi.org/10.1038/nrendo.2010.228
Fernandez-Real JM, Manco M (2014) Effects of iron overload on chronic metabolic diseases. Lancet Diabetes Endocrinol 2:513–526. https://doi.org/10.1016/S2213-8587(13)70174-8
Fuentes E, Venegas B, Munoz-Arenas G et al (2023) High-carbohydrate and fat diet consumption causes metabolic deterioration, neuronal damage, and loss of recognition memory in rats. J Chem Neuroanat 129:102237. https://doi.org/10.1016/j.jchemneu.2023.102237
García-Pardo MP, De la Rubia Ortí JE, Aguilar Calpe MA (2017) Differential effects of MDMA and cocaine on inhibitory avoidance and object recognition tests in rodents. Neurobiol Learn Mem 146:1–11. https://doi.org/10.1016/j.nlm.2017.10.013
Sekar G, G M, Krishnan M, Babu S, K P, Konduru P, S M, (2022) Effect of β-sitosterol on insulin resistance & protein expression of insulin signalling molecules in quadriceps muscle of high fat diet-induced type-2 diabetic rats. Bioinformation 18:1098–1104. https://doi.org/10.6026/973206300181098
Alkhalidy H, Al-Nabulsi A, Mhawish R, Liu D (2023) Low-dose of phenolic rich extract from Annona squamosa Linn leaves ameliorates insulin sensitivity and reduces body weight gain in HF diet-induced obesity. Front Nutr 10:1146021. https://doi.org/10.3389/fnut.2023.1146021
Aljahdali BA, Bajaber AS, Al-Nouri DM, Al-Khalifah AS, Arzoo S, Alasmari AA (2023) The development of nonalcoholic fatty liver disease and metabolic syndromes in diet-induced rodent models. Life (Basel) 13:1336. https://doi.org/10.3390/life13061336
Dongiovanni P, Ruscica M, Rametta R et al (2013) Dietary iron overload induces visceral adipose tissue insulin resistance. Am J Pathol 182:2254–2263. https://doi.org/10.1016/j.ajpath.2013.02.019
Kumar S, Senapati S, Bhattacharya N, Bhattacharya A, Maurya SK, Husain H, Bhatti JS, Pandey AK (2023) Mechanism and recent updates on insulin-related disorders. World J Clin Cases 11:5840–5856. https://doi.org/10.12998/wjcc.v11.i25.5840
Jodeiri Farshbaf M, Kiani-Esfahani A (2018) Succinate dehydrogenase: prospect for neurodegenerative diseases. Mitochondrion 42:77–83. https://doi.org/10.1016/j.mito.2017.12.002
Jais A, Solas M, Backes H et al (2016) Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell 165:882–895. https://doi.org/10.1016/j.cell.2016.03.033
Selman A, Burns S, Reddy AP, Culberson J, Reddy PH (2022) The role of obesity and diabetes in dementia. Int J Mol Sci 23:9267. https://doi.org/10.3390/ijms23169267
Kellar D, Craft S (2020) Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol 19:758–766. https://doi.org/10.1016/S1474-4422(20)30231-3
Gabbouj S, Ryhänen S, Marttinen M et al (2019) Altered insulin signaling in Alzheimer’s disease brain—special emphasis on PI3K-Akt pathway. Front Neurosci 13:629. https://doi.org/10.3389/fnins.2019.00629
Schubert M, Gautam D, Surjo D et al (2004) Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci USA 101:3100–3105. https://doi.org/10.1073/pnas.0308724101
Alves SS, Servilha-Menezes G, Rossi L, da Silva Junior RMP, Garcia-Cairasco N (2023) Evidence of disturbed insulin signaling in animal models of Alzheimer’s disease. Neurosci Biobehav Rev 152:105326. https://doi.org/10.1016/j.neubiorev.2023.105326
Acknowledgements
This work was supported by National Council for Scientific and Technological Development [CNPq; Grant numbers 403154/2021-9 and 305656/2019-8 to N.S.]; the National Institute of Science and Technology for Translational Medicine [INCT-TM—Grant number 465458/2014-9]; National Institute of Science and Technology for Brain Diseases, Excitotoxicity and Neuroprotection [INCT-EN—Grant number 465671/2014-4]; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. M.R.B, E.B., and N.S are Research Career Awardees of the CNPq. The funding sources were not involved in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
FVN: conceptualization, methodology, investigation, writing—original draft. MPP: investigation. BSF: investigation, resources, project administration. LK: investigation. CSS: investigation. LWK: investigation, validation. MRB: conceptualization, methodology, validation, writing—review and editing, funding acquisition. EB: conceptualization, methodology, validation, writing—review and editing, supervision, funding acquisition. NS: conceptualization, methodology, validation, formal analysis, writing—original draft, writing—review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the Institutional Ethics Committee for the Use of Animals of the Pontifical Catholic University (CEUA, #9331) and all experimental procedures were performed in accordance with the Brazilian Guidelines for the Care and Use of Animals in Research and Teaching (DBCA, published by CONCEA, MCTI, Brazil).
Consent for publication
All listed authors have approved the manuscript before submission, including the names and order of authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
do Nascimento, F.V., de Freitas, B.S., dos Passos, M.P. et al. A high fat diet potentiates neonatal iron overload-induced memory impairments in rats. Eur J Nutr 63, 1163–1175 (2024). https://doi.org/10.1007/s00394-024-03333-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-024-03333-x