Abstract
Background
In patients with chronic heart failure (CHF) and type 2 diabetes (T2D), sodium–glucose cotransporter-2 (SGLT2) inhibition improves cardiorenal outcomes, but details of the effects on distinct subsets of body fluid volume remain incomplete.
Methods
This was a post hoc analysis of patients with CHF and T2D in the CANDLE trial (UMIN000017669), an investigator-initiated, multi-center, randomized open-label trial that compared the effect of canagliflozin (100 mg, n = 113) with glimepiride (starting dose: 0.5 mg, n = 120) on changes in N-terminal pro-brain natriuretic peptide. The estimated plasma volume (ePV, calculated with the Straus formula) and estimated extracellular volume (eEV, determined by the body surface area) were compared between treatment groups at weeks 4, 12, and 24.
Results
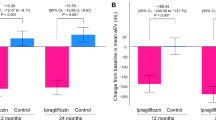
Among 233 patients analyzed, 166 (71.2%) had an ejection fraction (EF) > 50%. Reductions in ePV and eEV were observed only in the canagliflozin group until week 12 (change from baseline at week 12, ePV; − 7.63%; 95% confidence interval [CI], − 10.71 to − 4.55%, p < 0.001, eEV; − 123.15 mL; 95% CI, − 190.38 to − 55.92 mL, p < 0.001). While ePV stopped falling after week 12, eEV continued to fall until week 24 ([change from baseline at week 24] − [change from baseline at week 12], ePV; 1.01%; 95%CI, − 2.30–4.32%, p = 0.549, eEV; − 125.15 mL; 95% CI, − 184.35 to − 65.95 mL, p < 0.001).
Conclusions
Maintenance of a modest reduction in ePV and continuous removal of eEV via chronic SGLT2 inhibition suggests that favorable body fluid regulation contributes to the cardiorenal benefits of SGLT2 inhibitors in patients with CHF, irrespective of EF.
Trial Registration
UMIN000017669.
Graphical abstract




Similar content being viewed by others
Availability of data and materials
The datasets analyzed during the current study are available from the CANDLE study support office on reasonable request (candle-sub@clin-med.org).
Abbreviations
- BMI:
-
Body mass index
- CHF:
-
Chronic heart failure
- CI:
-
Confidence interval
- EF:
-
Ejection fraction
- ePV:
-
Estimated plasma volume
- eEV:
-
Estimated extracellular volume
- eGFR:
-
Estimated glomerular filtration rate
- GFR:
-
Glomerular filtration rate
- Hb:
-
Hemoglobin
- HbA1c:
-
Hemoglobin A1c
- HF:
-
Heart failure
- Ht:
-
Hematocrit
- LVEF:
-
Left-ventricular ejection fraction
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- NYHA:
-
New York Heart Association
- SBP:
-
Systolic blood pressure
- SGLT2:
-
Sodium–glucose cotransporter-2
- T2D:
-
Type 2 diabetes
References
Zelniker TA, Wiviott SD, Raz I et al (2019) SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393:31–39. https://doi.org/10.1016/s0140-6736(18)32590-x
Zannad F, Ferreira JP, Pocock SJ et al (2020) SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 396:819–829. https://doi.org/10.1016/s0140-6736(20)31824-9
McMurray JJV, Solomon SD, Inzucchi SE et al (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381:1995–2008. https://doi.org/10.1056/NEJMoa1911303
Packer M, Anker SD, Butler J et al (2020) Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383:1413–1424. https://doi.org/10.1056/NEJMoa2022190
Anker SD, Butler J, Filippatos G et al (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385:1451–1461. https://doi.org/10.1056/NEJMoa2107038
Tanaka A, Node K (2021) Fluid volume regulation in patients with heart failure. Lancet Diabetes Endocrinol 9:256–257. https://doi.org/10.1016/s2213-8587(21)00082-6
Jensen J, Omar M, Kistorp C et al (2021) Effects of empagliflozin on estimated extracellular volume, estimated plasma volume, and measured glomerular filtration rate in patients with heart failure (Empire HF Renal): a prespecified substudy of a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 9:106–116. https://doi.org/10.1016/s2213-8587(20)30382-x
Tanaka A, Shimabukuro M, Teragawa H et al (2021) Reduction of estimated fluid volumes following initiation of empagliflozin in patients with type 2 diabetes and cardiovascular disease: a secondary analysis of the placebo-controlled, randomized EMBLEM trial. Cardiovasc Diabetol 20:105. https://doi.org/10.1186/s12933-021-01295-6
Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW (2018) Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 20:479–487. https://doi.org/10.1111/dom.13126
Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC (2020) Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE-CHF trial. Circulation 142:1713–1724. https://doi.org/10.1161/circulationaha.120.048739
Boorsma EM, Ter Maaten JM, Damman K et al (2020) Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol 17:641–655. https://doi.org/10.1038/s41569-020-0379-7
Tanaka A, Inoue T, Kitakaze M et al (2016) Rationale and design of a randomized trial to test the safety and non-inferiority of canagliflozin in patients with diabetes with chronic heart failure: the CANDLE trial. Cardiovasc Diabetol 15:57. https://doi.org/10.1186/s12933-016-0381-x
Tanaka A, Hisauchi I, Taguchi I et al (2020) Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE). ESC Heart Fail 7:1585–1594. https://doi.org/10.1002/ehf2.12707
Duarte K, Monnez JM, Albuisson E, Pitt B, Zannad F, Rossignol P (2015) Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail 3:886–893. https://doi.org/10.1016/j.jchf.2015.06.014
Dekkers CCJ, Sjöström CD, Greasley PJ, Cain V, Boulton DW, Heerspink HJL (2019) Effects of the sodium–glucose co-transporter-2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes Metab 21:2667–2673. https://doi.org/10.1111/dom.13855
Christensen AB, Groth S (1986) Determination of 99mTc-DTPA clearance by a single plasma sample method. Clin Physiol 6:579–588. https://doi.org/10.1111/j.1475-097x.1986.tb00790.x
Matsuo S, Imai E, Horio M et al (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992. https://doi.org/10.1053/j.ajkd.2008.12.034
Gheorghiade M, Follath F, Ponikowski P et al (2010) Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 12:423–433. https://doi.org/10.1093/eurjhf/hfq045
Kociol RD, McNulty SE, Hernandez AF et al (2013) Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail 6:240–245. https://doi.org/10.1161/circheartfailure.112.969246
Lala A, McNulty SE, Mentz RJ et al (2015) Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from diuretic optimization strategy evaluation in acute decompensated heart failure (DOSE-AHF) and cardiorenal rescue study in acute decompensated heart failure (CARESS-HF). Circ Heart Fail 8:741–748. https://doi.org/10.1161/circheartfailure.114.001957
Aronson D, Abassi Z, Allon E, Burger AJ (2013) Fluid loss, venous congestion, and worsening renal function in acute decompensated heart failure. Eur J Heart Fail 15:637–643. https://doi.org/10.1093/eurjhf/hft036
Goldsmith SR, Bart BA, Burnett J (2014) Decongestive therapy and renal function in acute heart failure: time for a new approach? Circ Heart Fail 7:531–535. https://doi.org/10.1161/circheartfailure.113.000828
Ellison DH (2019) Clinical pharmacology in diuretic use. Clin J Am Soc Nephrol 14:1248–1257. https://doi.org/10.2215/cjn.09630818
Miller WL, Lobo R, Grill DE, Mullan BP (2021) Diuresis-related weight loss reflects interstitial compartment decongestion with minimal impact on intravascular volume expansion or outcomes in post-acute heart failure: metrics of decongestion and volume status. J Card Fail 27:445–452. https://doi.org/10.1016/j.cardfail.2020.12.006
Huang CY, Lin TT, Wu YF, Chiang FT, Wu CK (2019) Long-term prognostic value of estimated plasma volume in heart failure with preserved ejection fraction. Sci Rep 9:14369. https://doi.org/10.1038/s41598-019-50427-2
Matsuba I, Takihata M, Takai M et al (2021) Effects of 1-year treatment with canagliflozin on body composition and total body water in patients with type 2 diabetes. Diabetes Obes Metab 23:2614–2622. https://doi.org/10.1111/dom.14508
Matsubayashi Y, Yoshida A, Suganami H et al (2020) Association of increased hepatic insulin clearance and change in serum triglycerides or β-hydroxybutyrate concentration via the sodium/glucose-cotransporter 2 inhibitor tofogliflozin. Diabetes Obes Metab 22:947–956. https://doi.org/10.1111/dom.13980
Omar M, Jensen J, Frederiksen PH et al (2020) Effect of empagliflozin on hemodynamics in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 76:2740–2751. https://doi.org/10.1016/j.jacc.2020.10.005
Takagi K, Sato N, Ishihara S et al (2020) Differences in pharmacological property between combined therapy of the vasopressin V2-receptor antagonist tolvaptan plus furosemide and monotherapy of furosemide in patients with hospitalized heart failure. J Cardiol 76:499–505. https://doi.org/10.1016/j.jjcc.2020.05.012
Verma S, McMurray JJV (2018) SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 61:2108–2117. https://doi.org/10.1007/s00125-018-4670-7
Sawamura T, Karashima S, Nagase S et al (2020) Effect of sodium–glucose cotransporter-2 inhibitors on aldosterone-to-renin ratio in diabetic patients with hypertension: a retrospective observational study. BMC Endocr Disord 20:177. https://doi.org/10.1186/s12902-020-00656-8
Shin SJ, Chung S, Kim SJ et al (2016) Effect of sodium–glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS ONE 11:e0165703. https://doi.org/10.1371/journal.pone.0165703
Marenzi G, Lauri G, Grazi M, Assanelli E, Campodonico J, Agostoni P (2001) Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure. J Am Coll Cardiol 38:963–968. https://doi.org/10.1016/s0735-1097(01)01479-6
Acknowledgements
The authors thank all participants, investigators, board members, and medical staff involved in the CANDLE trial.
Funding
The work was funded by Mitsubishi Tanabe Pharma Corporation. The funders of the trial had no role in the study design, data collection, analysis or interpretation, or writing of the report.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception, design, and procedures. Funding acquisition for the study was carried out by KN, the principal investigator of the CANDLE trial. The data analyses were performed by SF, TakuI, AT, and KN. TakuI was responsible for the statistical analyses. The first draft of the manuscript was written by SF, and all authors reviewed subsequent drafts of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
SF received research funding from Boehringer Ingelheim and research grants from Bayer. AT received honoraria from Boehringer Ingelheim and research funding from GlaxoSmithKline and Takeda. TakuI received lecture fees from JCR Pharmaceuticals and Kyowa Kirin; and outsourcing fees from Organization for Clinical Medicine Promotion. MS received honoraria from Astellas, Boehringer Ingelheim, Mitsubishi Tanabe, and AstraZeneca. TM has received honoraria and a research grant from Mitsubishi Tanabe Pharma. TakaI received lecture honoraria from Otsuka Pharmaceutical Co. and Daiichi-Sankyo Pharmaceutical. NK has received honoraria from MSD, Astella, AstraZeneca, Novartis Pharma, Ono Pharmaceutical, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Boehringer Ingelheim Japan, Takeda Pharmaceutical; research grants from Asahi Kasei, Astellas, Mitsubishi Tanabe Pharma, Tei** Pharma, Terumo, Boehringer Ingelheim Japan, Eli Lilly and Company, Mochida Pharmaceutical, Fuji Yakuhin; and scholarships from Daiichi Sankyo Healthcare, Tei** Pharma, Medtronic, Bayer Yakuhin. All other authors declare no competing interests.
Ethics approval and consent to participate
The ethics committees of the participating institutions approved the study protocol. Written, informed consent for participation in the study was obtained from all subjects. This trial was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Consent for publication
All authors have read and approved the submission of the manuscript. The manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language. If the manuscript is accepted, we approve it for publication in Cardiovascular Diabetology.
Rights and permissions
About this article
Cite this article
Fujiki, S., Tanaka, A., Imai, T. et al. Body fluid regulation via chronic inhibition of sodium–glucose cotransporter-2 in patients with heart failure: a post hoc analysis of the CANDLE trial. Clin Res Cardiol 112, 87–97 (2023). https://doi.org/10.1007/s00392-022-02049-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-022-02049-4