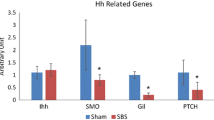
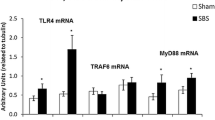
Abstract
Purpose
Nowadays, the standard therapy for patients with short bowel syndrome is parenteral nutrition (PN). Various growth factors have been tested to achieve weaning from prolonged PN administration. We evaluated the effect of hepatocyte growth factor (HGF) on structural intestinal adaptation and cell proliferation in a rat model of SBS.
Methods
Thirty Sprague–Dawley rats were divided into three groups; group A rats (sham) underwent bowel transection, group B rats underwent a 75% bowel resection, and group C rats underwent the same procedure but were treated postoperatively with HGF. Histopathologic parameters of intestinal adaptation were determined, while microarray and rt-PCR analyses of ileal RNA were also performed.
Results
Treatment with HGF resulted in significant increase in body weight, while the jejunal and ileal villus height and crypt depth were increased in HGF rats (36%, p < 0.05 and 27%, p < 0.05 respectively). Enterocyte proliferation was also significantly increased in HGF rats (21% p < 0.05). Microarray and quantitative rt-PCR analyses showed that the genes hgfac, rac 1, cdc42, and akt 1 were more than twofold up-regulated after HGF treatment.
Conclusion
HGF emerges as a growth factor that enhances intestinal adaptation. The future use of HGF may potentially reduce the requirement for PN in SBS patients.




Similar content being viewed by others
Data availability
All study results can be found in our institutional Databank and are also available at Figshare Data Repository.
References
Duggan CP, Jaksic T (2017) Pediatric Intestinal Failure. N Engl J Med 377:666–675. https://doi.org/10.1056/NEJMra1602650
Jeppesen PB, Pertkiewicz M, Messing B, Iyer K, Seidner DL, O’keefe SJ, et al (2012) Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 143:1473–81.e3. https://doi.org/10.1053/j.gastro.2012.09.007
Ukleja A (2019) Weaning from Parenteral Nutrition. Gastroenterol Clin North Am 48:525–550. https://doi.org/10.1016/j.gtc.2019.08.007
McMellen ME, Wakeman D, Longshore SW, McDuffie LA, Warner BW (2010) Growth factors: possible roles for clinical management of the short bowel syndrome. Semin Pediatr Surg 19:35–43. https://doi.org/10.1053/j.sempedsurg.2009.11.010
Billiauws L, Joly F (2019) Emerging treatments for short bowel syndrome in adult patients. Expert Rev Gastroenterol Hepatol 13:241–246. https://doi.org/10.1080/17474124.2019.1569514
Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS (2004) Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A 101:4477–4482. https://doi.org/10.1073/pnas.0306068101
Organ SL, Tsao MS (2011) An overview of the c-MET signaling pathway. Ther Adv Med Oncol 3(1 Suppl):S7–S19. https://doi.org/10.1177/1758834011422556
Sugita K, Kaji T, Yano K, Matsukubo M, Nagano A, Matsui M, Murakami M, Harumatsu T, Onishi S, Yamada K, Yamada W, Muto M, Kumagai K, Ido A, Ieiri S (2021) The protective effects of hepatocyte growth factor on the intestinal mucosal atrophy induced by total parenteral nutrition in a rat model. Pediatr Surg Int 37:1743–1753. https://doi.org/10.1007/s00383-021-05002-0
Mizuno S, Nakamura T (2007) Hepatocyte growth factor: a regenerative drug for acute hepatitis and liver cirrhosis. Regen Med 2:161–170. https://doi.org/10.2217/17460751.2.2.161
Yano K, Sugita K, Muto M, Matsukubo M, Onishi S, Kedoin C, Matsui M, Murakami M, Harumatsu T, Yamada K, Yamada W, Kumagai K, Ido A, Kaji T, Ieiri S (2022) The preventive effect of recombinant human hepatocyte growth factor for hepatic steatosis in a rat model of short bowel syndrome. J Pediatr Surg 57:1286–1292. https://doi.org/10.1016/j.jpedsurg.2022.02.030
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al (2020). The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. doi: https://doi.org/10.1371/journal.pbio.3000410
Thatch KA, Schwartz MZ, Yoo EY, Mendelson KG, Duke DS (2008) Modulation of the inflammatory response and apoptosis using epidermal growth factor and hepatocyte growth factor in a liver injury model: a potential approach to the management and treatment of cholestatic liver disease. J Pediatr Surg 43:2169–2173. https://doi.org/10.1016/j.jpedsurg.2008.08.045
Kato Y, Yu D, Lukish JR, Schwartz MZ (1997) Hepatocyte growth factor enhances intestinal mucosal cell function and mass in vivo. J Pediatr Surg 32:991–994. https://doi.org/10.1016/s0022-3468(97)90384-5
Misiakos EP, Agrogiannis G, Patapis P, Dontas I, Petropoulos K, Machairas N, Giamarellos-Bourboulis E, Liakakos T, Machairas A (2013) Expression of tissue IGF 1, TGFbeta and EGFR in the sequential steps of intestinal adaptation in a rat model of short bowel syndrome. Acta Chir Belg 113:129–138
Sukhotnik I, Haj B, Pollak Y, Dorfman T, Bejar J, Matter I (2016) Effect of bowel resection on TLR signaling during intestinal adaptation in a rat model. Surg Endosc 30(10):4416–4424. https://doi.org/10.1007/s00464-016-4760-x
Sukhotnik I, Razon H, Pollak Y, Hayari L, Bejar J, Mogilner JG, Sylvester KG (2012) Effect of alpha-naphthylisothiocyanate-induced liver injury on intestinal adaptation in a rat model of short bowel syndrome. Pediatr Surg Int 28:161–169. https://doi.org/10.1007/s00383-011-2989-y
Nakamura T, Nawa K, Ichihara A (1984) Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun 122:1450–1459
Russell WE, McGowan JA, Bucher NL (1984) Partial characterization of a hepatocyte growth factor from rat platelets. J Cell Physiol 119:183–192
Miyazawa K, Tsubouchi H, Naka D et al (1989) Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun 163:967–973
Kataoka H, Kawaguchi M (2010) Hepatocyte growth factor activator (HGFA): pathophysiological functions in vivo. FEBS J 277:2230–2237. https://doi.org/10.1111/j.1742-4658.2010.07640.x
Nakamura T, Nishizawa T, Hagiya M et al (1989) Molecular cloning and expression of human hepatocyte growth factor. Nature 342:440–443
Comoglio PM, Giordano S, Trusolino L (2008) Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 7:504–516. https://doi.org/10.1038/nrd2530
Misiakos EP, Kato T, Levi D, et al (2000) Pediatric small bowel transplantation. In: Tejani A, Harmon WE, Fine R, eds. Pediatric Solid Organ Transplantation. Munksgaard,
Levin MS, Rubin DC (2008) Intestinal adaptation. The biology of the intestinal response to resection and disease. In: A. Intestinal failure. Diagnosis, management and transplantation. Blackwell Publishing, Inc., Malden MA, pp. 45–54.
Wilson TJ, Ponder BA, Wright NA (1985) Use of a mouse chimaeric model to study cell migration patterns in the small intestinal epithelium. Cell Tissue Kinet 18:333–344
Pinto D, Clevers H (2005) Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 306:357–363
Okamoto R, Tsuchiya K, Nemoto Y, Akiyama J, Nakamura T, Kanai T, Watanabe M (2009) Requirement of Notch activation during regeneration of the intestinal epithelia. Am J Physiol Gastrointest Liver Physiol 296:G23–G35. https://doi.org/10.1152/ajpgi.90225.2008
van den Brink GR (2007) Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev 87:1343–1375. https://doi.org/10.1152/physrev.00054.2006
Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK (2004) Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 126:796–808. https://doi.org/10.1053/j.gastro.2003.12.004
Misiakos EP, Weppler D, Bakonyi A, Nery JR, Pinna AD, Kato T, Rodriguez M, Ruiz P, Thompson J, Ricordi C, Tzakis AG (1999) Clinical outcome of intestinal transplantation at the University of Miami. Transplant Proc 31:569–571. https://doi.org/10.1016/s0041-1345(98)01558-9
Sukhotnik I, Mogilner JG, Pollak Y, Blumenfeld S, Bejar J, Coran AG (2012) PDGF-α stimulates intestinal epithelial cell turnover after massive small bowel resection in a rat. Am J Physiol Gastrointest Liver Physiol 302:G1274–G1281. https://doi.org/10.1152/ajpgi.00532.2011
Williamson RC, Buchholtz TW, Malt RA (1978) Humoral stimulation of cell proliferation in small bowel after transection and resection in rats. Gastroenterology 75:249–254
Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA (2007) Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. American Journal of Physiology-Gastrointestinal and Liver Physiology 293:G1013-G1022. doi: https://doi.org/10.1152/ajpgi.00218.2007.
Haxhija EQ, Yang H, Spencer AU, Sun X, Teitelbaum DH (2006) Influence of the site of small bowel resection on intestinal epithelial cell apoptosis. Pediatr Surg Int 22:37–42. https://doi.org/10.1007/s00383-005-1576-5
Wang HT, Miller JH, Avissar N, Sax HC (1999) Small bowel adaptation is dependent on site of massive enterectomy. J Surg Res 84:94–100. https://doi.org/10.1006/jsre.1999.5616
Falcone RA, Stern LE, Kemp CJ, Shin CE, Erwin CR, Warner BW (1999) Apoptosis and the pattern of DNase I expression following massive small bowel resection. J Surg Res 84:218–222. https://doi.org/10.1006/jsre.1999.5649
**ao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR (2001) Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A 98:247–252. https://doi.org/10.1073/pnas.011532898
Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C (2009) STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206:1465–1472. https://doi.org/10.1084/jem.20082683
Bierne H, Cossart P (2002) InlB, a surface protein of Listeria monocytogenes that behaves as an invasin and a growth factor. J Cell Sci 115:3357–3367
Hall A (1998) Rho GTPases and the actin cytoskeleton. Science 279:509–514
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB (1999) NFkappaB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature 401:82–85
Feng Y, Demehri FR, **ao W et al (2017) Interdependency of EGF and GLP-2 Signaling in Attenuating Mucosal Atrophy in a Mouse Model of Parenteral Nutrition. Cell Mol Gastroenterol Hepatol 3:447–468. https://doi.org/10.1016/j.jcmgh.2016.12.005
Kato Y, Yu D, Schwartz MZ (1998) Enhancement of intestinal adaptation by hepatocyte growth factor. J Pediatr Surg 33:235–239. https://doi.org/10.1016/s0022-3468(98)90438-9
Katz MS, Thatch KA, Schwartz MZ (2011) Chronology of the effect of massive small bowel resection and hepatocyte growth factor (HGF) on intestinal adaptation. J Surg Res 171:399–403. https://doi.org/10.1016/j.jss.2011.04.007
Kinoshita T, Hirao S, Matsumoto K, Nakamura T (1991) Possible endocrine control by hepatocyte growth factor of liver regeneration after partial hepatectomy. Biochem Biophys Res Commun 177:330–335. https://doi.org/10.1016/0006-291x(91)91987-n
Ogura Y, Hamanoue M, Tanabe G et al (2001) Hepatocyte growth factor promotes liver regeneration and protein synthesis after hepatectomy in cirrhotic rats. Hepatogastroenterology 48:545–549
Liu J, Pan G, Liang T, Huang P (2014) HGF/c-Met signaling mediated mesenchymal stem cell-induced liver recovery in intestinal ischemia reperfusion model. Int J Med Sci 11:626–633. https://doi.org/10.7150/ijms.8228
Barron LK, Bao JW, Aladegbami BG, Colasanti JJ, Guo J, Erwin CR, Warner BW (2017) Toll-like receptor 4 is critical for the development of resection-associated hepatic steatosis. J Pediatr Surgery 52:1014–1019. https://doi.org/10.1016/j.jpedsurg.2017.03.026
Bradbury J (1998) A two-pronged approach to the clinical use of HGF. Lancet 351:272. https://doi.org/10.1016/S0140-6736(05)78259-3
Ido A, Moriuchi A, Numata M, Murayama T, Teramukai S, Marusawa H, Yamaji N, Setoyama H, Kim ID, Chiba T, Higuchi S, Yokode M, Fukushima M, Shimizu A, Ido TH, A., Moriuchi, A., Numata, M., Murayama, T., Teramukai, S., Marusawa, H., Yamaji, N., Setoyama, H., Kim, I. D., Chiba, T., Higuchi, S., Yokode, M., Fukushima, M., Shimizu, A., & Tsubouchi, H. (2011) Safety and pharmacokinetics of recombinant human hepatocyte growth factor (rh-HGF) in patients with fulminant hepatitis: a phase I/II clinical trial, following preclinical studies to ensure safety. J Transl Med 9:55. https://doi.org/10.1186/1479-5876-9-55
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Animal care and operative procedures were performed by George Bagias, Evangelos Misiakos and Evangelos Giamarelos—Bourboulis. Tissue preparation and histological analysis were performed by Stratigoula Sakellariou. Microarray analysis was performed by Igor Sukhotnik and rt-PCR analysis was performed by George Bagias. Data collection and analysis were performed by George Bagias, Anestis Charalampopoulos, and Nick Zavras. The first draft of the manuscript was written by George Bagias and Evangelos Misiakos, Dimitrios Schizas, and Emmanouil Pikoulis commented on previous versions of the manuscript and conducted the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
The study has been approved by the Veterinary Department of the Region of Attica under the code 441/22–01-2016 and the local ethics committee under the code 1281/1–2-16. Animal care complied with the guide for the care and use of laboratory animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bagias, G., Misiakos, E.P., Charalampopoulos, A. et al. The effect of hepatocyte growth factor on intestinal adaption in an experimental model of short bowel syndrome. Pediatr Surg Int 39, 80 (2023). https://doi.org/10.1007/s00383-022-05341-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-022-05341-6