Abstract
Context
There is an urgent need to develop novel treatment strategies in patients with unfavorable intermediate- and high-risk localized prostate cancer (PCa) to optimize the outcome of these patients. Androgen receptor signaling inhibitors (ARSI) have demonstrated a survival benefit in metastatic hormonesensitive and castration-resistant PCa. A similar benefit might be expected in the localized setting.
Objective
To perform a systematic review about the role of neoadjuvant ARSI in unfavorable intermediate and high-risk localized PCa.
Evidence acquisition
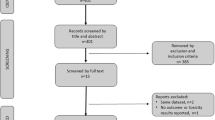
We performed a systematic review of the following databases: MEDLINE (PubMed), EMBASE, Cochrane Library and Web of Science. Publications of ASCO were consulted to identify meeting abstract with early results of ongoing trials. This systematic review was performed and reported in accordance with the PRISMA guidelines.
Evidence synthesis
Pathological complete response (pCR) following neoadjuvant ARSI treatment was observed in 4%–13% of the patients. Minimal residual disease response ranged from 36% to 73.9% when defined as residual cancer burden < 0.25 cm3 at final pathology and from 8% to 20% when defined as the diameter of the remaining tumor < 5 mm. Despite intense neoadjuvant ARSI treatment, residual pT3 disease was observed in 48%–76% of the patients. In contrast, positive surgical margins (PSM) were present in only 5%–22%. Only one trial reported BCR following neoadjuvant ARSI therapy (44% BCR at a median follow-up of 4 years).
Conclusion
Despite intense neoadjuvant ARSI therapy, pCR is rarely attained and high proportions of pT3 disease are still observed at final pathology. In contrast, promising results are obtained in terms of PSMs. Long-term survival outcomes are eagerly awaited.
Similar content being viewed by others
References
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Cooperberg MR, Broering JM, Carroll PR (2010) Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol 28:1117–1123
D’Amico AV et al (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969–974
Van den Broeck T et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur. Urol. 0, (2018).
Shelley MD et al (2009) A systematic review and meta-analysis of randomised trials of neo-adjuvant hormone therapy for localised and locally advanced prostate carcinoma. Cancer Treat Rev 35:9–17
Tucci M et al (2019) Therapeutic options for first-line metastatic castration-resistant prostate cancer: Suggestions for clinical practise in the CHAARTED and LATITUDE era. Cancer Treat Rev 74:35–42
Kinsey EN, Zhang T, Armstrong AJ (2020) Metastatic hormone-sensitive prostate cancer: a review of the current treatment landscape. Cancer J (United States) 26:64–75
Efstathiou E et al (2019) Clinical and biological characterisation of localised high-risk prostate cancer: results of a randomised preoperative study of a luteinising hormone-releasing hormone agonist with or without abiraterone acetate plus prednisone. Eur Urol 76:418–424
McKay RR et al (2019) Evaluation of intense androgen deprivation before prostatectomy: a randomized phase II trial of enzalutamide and leuprolide with or without abiraterone. J. Clin. Oncol. JCO.18.01777. https://doi.org/10.1200/JCO.18.01777.
Montgomery B et al (2017) Neoadjuvant enzalutamide prior to prostatectomy. Clin Cancer Res 23:2169–2176
Taplin M-E et al (2014) Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol 32:3705–3715
Efstathiou E et al (2020) Neoadjuvant apalutamide (APA) plus leuprolide (LHRHa) with or without abiraterone (AA) in localized high-risk prostate cancer (LHRPC). J Clin Oncol 38:5504–5504
McKay RR et al (2020) Results of a phase II trial of intense androgen deprivation therapy prior to radical prostatectomy (RP) in men with high-risk localized prostate cancer (PC). J Clin Oncol 38:5503–5503
Cortazar P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164–172
Mckay RR et al (2018) Post prostatectomy outcomes of patients with high-risk prostate cancer treated with neoadjuvant androgen blockade. Prostate Cancer Prostatic Dis 21:364–372
Page ST et al (2006) Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab 91:3850–3856
Mostaghel EA et al (2007) Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res 67:5033–5041
Beltran H et al (2017) Impact of therapy on genomics and transcriptomics in high-risk prostate cance treated with neoadjuvant docetaxel and androgen deprivation therapy. Clin Cancer Res 23:6802–6811
Corcoran N et al (2015) The predictive value of ARv7 expression in localized prostate caner treated with abiraterone, degarelix, and bicalutamide. J Clin Oncol 33:71–71
Sowalsky AG et al (2018) Neoadjuvant-intensive androgen deprivation therapy selects for prostate tumor foci with diverse subclonal oncogenic alterations. Cancer Res 78:4716–4730
Arora VK et al (2013) Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155:1309–1322
Wyatt AW et al (2016) Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol 2:1598–1606
De Laere B et al (2019) TP53 outperforms other androgen receptor biomarkers to predict abiraterone or enzalutamide outcome in metastatic castration-resistant prostate cancer. Clin Cancer Res 25:1766–1773
Mateo J et al. Genomics of lethal prostate cancer at diagnosis and castration-resistance. J Clin Invest 130, (2019).
Castro E et al (2013) Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 31:1748–1757
Marshall CH et al (2019) Prevalence of DNA repair gene mutations in localized prostate cancer according to clinical and pathologic features: association of Gleason score and tumor stage. Prostate Cancer Prostatic Dis 22:59–65
Isaacsson Velho P et al (2019) Molecular characterization and clinical outcomes of primary gleason pattern 5 prostate cancer after radical prostatectomy. JCO Precis Oncol 3:1–13
Eastham JA et al (2020) Cancer and leukemia group B 90203 (Alliance): radical prostatectomy with or without neoadjuvant chemohormonal therapy in localized, high-risk prostate cancer. J Clin Oncol 38:3042–3050
Serritella A et al (2020) Phase I/II trial of enzalutamide (Enz) plus mifepristone (Mif) for metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 38:91–91
De Bono JS et al (2019) Randomized phase II study evaluating AKT blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res 25:928–936
Ku SY, Gleave ME, Beltran H (2019) Towards precision oncology in advanced prostate cancer. Nat Rev Urol 16:645–654
de Bono J et al (2020) Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091–2102
Acknowledgements
Gaëtan Devos is a PhD fellow of the Research Foundation Flanders (FWO). Wout Devlies is a recipient of Emmanuel van der Schueren scholarship of “Kom Op Tegen Kanker”. Steven Joniau is a Senior Clinical Investigator of the FWO. The authors wish to thank Thomas Vandendriessche, Kristel Paque and Krizia Tuand, the biomedical reference librarians of the KU Leuven Libraries—2Bergen—learning Centre Désiré Collen (Leuven, Belgium), for their help in conducting the systematic literature search
Funding
This research received no external funding. This research was supported by the ‘Jozef De Wever’ fund for prostate cancer prevention of the KU Leuven, Leuven, Belgium.
Author information
Authors and Affiliations
Contributions
Conceptualization, GD, BV, SJ; writing—original draft preparation, GD, BV, SJ; writing—review and editing, All authors; supervision, SJ; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Devos, G., Vansevenant, B., De Meerleer, G. et al. Neoadjuvant treatment with androgen receptor signaling inhibitors prior to radical prostatectomy: a systematic review. World J Urol 39, 3177–3185 (2021). https://doi.org/10.1007/s00345-021-03611-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-021-03611-x