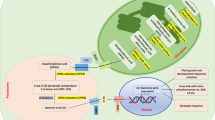
Abstract
A precise apprehension of natural products and their role in the growth and protection of plants is the key to predicate the development of novel products for enhanced plant production, both in vivo as well as in vitro. Over the years, polyamines (PAs) constitute the prime members of ubiquitously appearing natural plant products, tangled with a variety of plant functions ranging from inducing organogenesis to mitigating stress. Various pathways for PA biosynthesis have been elucidated in plants, animals, and micro-organisms stipulating the presence of some PA representatives in both eukaryotes and prokaryotes systems. PAs are low molecular weight diamines that mediate the basal metabolism of plants by regulating various cellular processes including molecular signaling, cell division and differentiation, totipotency, oxidative damage, and various stress responses. Conjugation of PAs with other small molecules like phenolic acids and other cellular components often leads to the accumulation of incongruous interaction between plants and pathogens. PAs thwarts the various plant stress responses by diminishing the toxicity symptoms, modulating the stomatal closure and antioxidant capacity, increasing the germination efficiency, imputing tolerance against the bacterial pathogens, and reducing the viral multiplication. This study explored and summarized the significance of the correlation between different PAs and diverse physiological parameters in plants and supplemented the functioning of PAs under harsh environmental conditions in plants.




Similar content being viewed by others
References
Abbasi NA, Ali I, Hafiz IA, Alenazi MM, Shafiq M (2019) Effects of putrescine application on peach fruit during storage. Sustainability 11:2013
Abdel G, Nahed A, Taha Lobna S, Soad MMI (2009) Some studies on the effect of putrescine, ascorbic acid and thiamine on growth, flowering and some chemical constituents of gladiolus plants at Nubaria. Ozean J Appl Sci 2(2):169–179
Adkins SW, Samosir YM, Nikmatullah A, Ogle H (2001) Coconut (Cocos nucifera) in vitro ecology: modifications of headspace and medium additives can optimize somatic embryogenesis. In: international symposium on biotechnology of tropical and subtropical species 692 pp. 21–32
Agurla S, Gayatri G, Raghavendra AS (2018) Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 255:153–162
Ahangari H, Kurbanoglu S, Ehsani A, Uslu B (2021) Latest trends for biogenic amines detection in foods: enzymatic biosensors and nanozymes applications. Trends Food Sci Technol 112:75–87
Ajithan C, Vasudevan V, Sathish D, Sathish S, Krishnan V, Manickavasagam M (2019) The influential role of polyamines on the in vitro regeneration of pea (Pisum sativum L.) and genetic fidelity assessment by SCoT and RAPD markers. Plant Cell Tissue Organ Cult 139:547–561. https://doi.org/10.1007/s11240-019-01699-z
Alcázar R, Cuevas JC, Patrón M, Altabella T, Tiburcio AF (2006a) Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiol Plant 128:448–455
Alcázar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T (2006b) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28:1867–1876. https://doi.org/10.1007/S10529-006-9179-3
Alcazar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Alcázar R, Bueno M, Tiburcio AF (2020) Polyamines: small amines with large effects on plant abiotic stress tolerance. Cells 9(11):2373
Alharbi B, Hunt JD, Dimitrova S, Spadafora ND, Cort AP, Colombo D, Müller CT, Ghuge SA, Davoli D, Cona A, Mariotti L (2020) Mutation of Arabidopsis copper-containing amine oxidase gene AtCuAOδ alters polyamines, reduces gibberellin content and affects development. Inter Jour Mol Sci 21(20):77–89
Ali RM, Abbas HM, Kamal RK (2009) The effects of treatment with polyamines on dry matter and some metabolites in salinity—stressed chamomile and sweet majoram seedlings. Plant Soil Environ 55:477–483
Amin AA, Gharib FAE, El-Awadi M, Rashad ESM (2011) Physiological response of onion plants to foliar application of putrescine and glutamine. Sci Hortic 129:353–360. https://doi.org/10.1016/j.scienta.2011.03.052
Amooaghaie R, Moghym S (2011) Effect of polyamines on thermotolerance and membrane stability of soybean seedling. Afr J Biotechnol 10:9673–9679
Angelini R, Cona A, Federico R, Fincato P, Tavladoraki P, Tisi A (2010) Plant amine oxidases “on the move”: an update. Plant Physiol Biochem 48:560–564
Anjum SA, Ashraf U, Tanveer M, Khan I, Hussain S, Shahzad B, Zohaib A, Saleen MF, Ali I, Wang LC (2017) Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front Plant Sci 8:69
Anwar R, Mattoo AK, Handa AK (2015) Polyamine interactions with plant hormones: crosstalk at several levels. In: Kusano T, Suzuki H (eds) Polyamines. Springer, Tokyo, pp 267–302. https://doi.org/10.1007/978-4-431-55212-3_22
Applewhite PB, Kaur-Sawhney R, Galston AW (2000) A role for spermidine in the bolting and flowering of Arabidopsis. Physiol Plant 108:314–320. https://doi.org/10.1034/j.1399-3054.2000.108003314.x
Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Kubis J (2009) Interaction between polyamine and nitric oxide signalling in adaptive responses to drought in cucumber. J Plant Growth Reg 28:177–186
Arena ME, Pastur GM, Benavides P, Curvetto N (2010) Polyamines and inhibitors used in successive culture media for in vitro rooting in Berberis buxifolia. N Z J Bot 43:373–380. https://doi.org/10.1080/0028825X.2005.9512962
Arun M, Subramanyam K, Theboral J, Ganapathi A, Manickavasagam M (2014) Optimized shoot regeneration for Indian soybean: the influence of exogenous polyamines. Plant Cell Tissue Organ Cult 117:305–309. https://doi.org/10.1007/s11240-014-0431-6
Asano K, Sasaki Y, Zhou Q, Mitobe R, Tang W, Lyu X, Kamiko M, Tanaka H, Yamagami A, Hagiya K, Minami T (2021) Detection of polyamines by an extended gate-type organic transistor functionalized with a carboxylate attached 1, 3, 4-thiadiazole derivative. J Mater Chem C 9:11690–11697
Ayad HS, Reda F, Abdalla MS (2010) Effect of putrescine and zinc on vegetative growth, photosynthetic pigments, lipid peroxidation and essential oil content of geranium (Pelargonium graveolens L.). World J Agric Sci 6:601–608
Aydin M, Pour AH, Haliloğlu K, Tosun M (2016) Effect of polyamines on somatic embryogenesis via mature embryo in wheat. Turkish J Biol 40(6):1178–1184. https://doi.org/10.3906/biy-1601-21
Bagni N, Tassoni A (2001) Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 20:301–317
Bais HP, Ravishankar GA (2002) Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tissue Organ Cult 69:1–34
Balci M, Alp FN, Arikan B, Ozfidan-Konakci C, Yildiztugay E (2022) Polyamine cadaverine detoxifies nitrate toxicity on the chloroplasts of Triticum aestivum through improved gas exchange, chlorophyll a fluorescence and antioxidant capacity. J Plant Growth Regul 1:1–17
Bhatnagar P, Minocha R, Minocha SC (2002) Genetic manipulation of the metabolism of polyamines in poplar cells. The regulation of putrescine catabolism. Plant Physiol 128:1455–1469
Bitrián M, Zarza X, Altabella T, Tiburcio AF, Alcázar R (2012) Polyamines under abiotic stress: metabolic crossroads and hormonal crosstalks in plants. Metabolites 2(3):516–528
Bunsupa S, Katayama K, Ikeura E, Oikawa A, Toyooka K, Saito K, Yamazaki M (2012) Lysine decarboxylase catalyzes the first step of quinolizidine alkaloid biosynthesis and coevolved with alkaloid production in leguminosae. Plant Cell 24:1202–1216
Cai G, Sobieszczuk-Nowicka E, Aloisi I, Fattorini L, Serafini-Fracassini D, Del Duca S (2015) Polyamines are common players in different facets of plant programmed cell death. Amino Acids 47(1):27–44
Cao DD, Hu J, Zhu SJ, Hu WM, Knapp A (2010) Relationship between changes in endogenous polyamines and seed quality during development of sh2 sweet corn (Zea mays L.) seed. Sci Hortic 123(3):301–307
Chambhare MR, Nikam TD (2022) Influence of plant growth regulators on somatic embryogenesis in Niger (Guizotia abyssinica Cass.): an edible oilseed crop. J Crop Sci Biotechnol 25:225–232
Champa HWA, Gill MIS, Mahajan BVC, Bedi S (2015) Exogenous treatment of spermine to maintain quality and extend postharvest life of table grapes (Vitis vinifera L.) cv. Flame seedless under low temperature storage. LWT Food Sci Technol 60:412–419. https://doi.org/10.1016/j.lwt.2014.08.044
Chen D, Shao Q, Yin L, Younis A, Zheng B (2019) Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci 9:1945
Cheng L, Zou Y, Ding S, Zhang J, Yu X, Cao J, Lu G (2009) Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J Integr Plant Biol 51:489–499
Cheon-Chae S (2016) Shoot organogenesis of Echinacea angustifolia DC as influenced by polyamines. Life Sci J 13:1097–8135. https://doi.org/10.7537/marslsj130116.03
Chong-Pérez B, Reyes M, Rojas L, Ocaña B, Pérez B, Kosky RG, Angenon G (2012) Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in banana cv. “Dwarf Cavendish” (Musa AAA): effect of spermidine on transformation efficiency. Plant Cell Tissue Organ Cult 111:79–90. https://doi.org/10.1007/s11240-012-0174-1
Chourasia KN, More SJ, Kumar A, Kumar D, Singh B, Bhardwaj V, Kumar A, Das SK, Singh RK, Zinta G, Tiwari RK (2022) Salinity responses and tolerance mechanisms in underground vegetable crops: an integrative review. Planta 255(3):1–25
Chunthaburee S, Sanitchon J, Pattanagul W, Theerakulpisut P (2014) Alleviation of salt stress in seedlings of black glutinous rice by seed priming with spermidine and gibberellic acid. Not Bot Hortic Agrobot 42:405–4013
Couée I, Hummel I, Sulmon C, Gouesbet G, El Amrani A (2004) Involvement of polyamines in root development. Plant Cell Tissue Organ Cult 76:1–10
Cuevas JC, López-Cobollo R, Alcázar R, Zarza X, Koncz C, Altabella T, Salinas J, Tiburcio AF, Ferrando A (2008) Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiol 148:1094–1105
Cui J, Pottosin I, Lamade E, Tcherkez G (2020) What is the role of putrescine accumulated under potassium deficiency? Plant Cell Environ 43:1331–1347. https://doi.org/10.1111/pce.13740
De la Torre-González A, Montesinos-Pereira D, Blasco B, Ruiz JM (2018) Influence of the proline metabolism and glycine betaine on tolerance to salt stress in tomato (Solanum lycopersicum L.) commercial genotypes. J Plant Physiol 231:329–336
de Oliveira LF, Navarro BV, Cerruti GV, Elbl P, Minocha R, Minocha SC, Dos Santos AL, Floh EI (2018) Polyamine- and amino acid-related metabolism: the roles of arginine and ornithine are associated with the embryogenic potential. Plant Cell Physiol 59:1084–1098. https://doi.org/10.1093/pcp/pcy049
Dey A, Hazra AK, Nongdam P, Nandy S, Tikendra L, Mukherjee A, Banerjee S, Mukherjee S, Pandey DK (2019) Enhanced bacoside content in polyamine treated in vitro raised Bacopa monnieri (L.) Wettst. S Afr J Bot 123:259–269. https://doi.org/10.1016/j.sajb.2019.03.012
Diao Q, Song Y, Shi D, Qi H (2017) Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum Mill.) seedlings. Front Plant Sci 8:203
Diwan R, Malpathak N (2008) Novel technique for scaling up of micropropagated Ruta graveolens shoots using liquid culture systems: a step towards commercialization. New Biotech 25:85–91
Do PT, Drechsel O, Heyer AG, Hincha DK, Zuther E (2014) Changes in free polyamine levels, expression of polyamine biosynthesis genes, and performance of rice cultivars under salt stress: a comparison with responses to drought. Front Plant Sci 5:182
Du H, Liu D, Liu G, Liu H, Sun H, Li C, Kurtenbach R (2022) Conjugated polyamines are involved in conformation stability of plasma membrane from maturing maize grain embryos under drought stress. Environ Exp Bot 194:104726
Duan J, Li J, Guo S, Kang Y (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165:1620–1635
Duan Q-L, Shi H-W, Tan L, Liu Z, Huang Q, Shen W, Cao L, Lee HK, Tang S (2022) Ultrahigh-performance supercritical fluid chromatography and detection of multiple biogenic amines in gentamicin sulfate: method development using computer-assisted modeling. Anal Chem. https://doi.org/10.1021/acs.analchem.2c00325
Dudley HW, Rosenheim O, Starling WW (1926) The chemical constitution of spermine: structure and synthesis. Biochem J 20:1082
Ebeed HT, Hassan NM, Aljarani AM (2017) Exogenous applications of polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol Biochem 118:438–448
Ebrahimzadeh H, Shariatpanahi ME, Ahmadi B, Soltanloo H, Lotfi M, Zarifi E (2018) Efficient parthenogenesis induction and in vitro haploid plant regeneration in cucumber (Cucumis sativus L.) using putrescine, spermidine, and cycocel. J Plant Growth Regul 37:1127–1134. https://doi.org/10.1007/s00344-018-9803-1
El-Dawayati M, Sayed Ghazzawy H, Munir M (2018) Somatic embryogenesis enhancement of date palm cultivar Sewi using different types of polyamines and glutamine amino acid concentration under in vitro solid and liquid media conditions. Int J Biosci 12:149–159. https://doi.org/10.12692/ijb/12.1.149-159
Fan J, Feng Z, Chen N (2020) Spermidine as a target for cancer therapy. Pharmacol Res 159:104943. https://doi.org/10.1016/j.phrs.2020.104943
Fang W, Qui F, Yin Y, Yang Z (2020) Exogenous spermidine promotes γ-aminobutyric acid accumulation and alleviates the negative effect of NaCl stress in germinating soybean (Glycine max L.). Foods 9:267
Farooq M, Wahid A, Lee DJ (2009) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945
Fazilati M, Forghani AH (2015) The role of polyamine to increasing growth of plant: as a key factor in health crisis. Int J Heal Syst Disaster Manag 3:89
Felix H, Harr J (1987) Association of polyamines to different parts of various plant species. Physiol Plant 71:245–250
Freschi L (2013) Nitric oxide and phytohormone interactions: current status and perspectives. Front Plant Sci 4:398
Fu X-Z, Chen C-W, Wang Y, Liu JH, Moriguchi T (2011) Ectopic expression of MdSPDS1 in sweet orange (Citrus sinensis Osbeck) reduces canker susceptibility: involvement of H2O2 production and transcriptional alteration. BMC Plant Biol 11:1–15
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525
Golldack D, Li C, Mohan H, Probst N (2014) Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci 5:151
González-García MP, Vilarrasa-Blasi J, Zhiponova M, Divol F, Mora-García S, Russinova E, Caño-Delgado AI (2011) Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 138(5):849–859
González-Hernández AI, Scalschi L, Vicedo B, Marcos-Barbero EL, Morcuende R, Camaes G (2022) Putrescine: a key metabolite involved in plant development, tolerance and resistance responses to stress. Int J Mol Sci 23:2971
Gross EM, Lowry ER, Schaffer LV, Henry CS (2022) Electrogenerated chemiluminescent detection of polyamines on a microfluidic device using micromolded carbon paste microelectrodes. Electroanalysis. https://doi.org/10.1002/elan.202100410
Guen-Le Saos L, Hourmant A (2001) Stimulation of putrescine biosynthesis via the ornithine decarboxylase pathway by gibberellic acid in the in vitro rooting of globe artichoke (Cynara scolymus). Plant Growth Regul 35:277–284
Guo JE, Li T, Sun X, Zheng C, Sun X (2015) Relationship between endogenous polyamines and floral bud differentiation in Chrysanthemum morifolium under short-day conditions. J Hortic Sci Technol 33:31–38. https://doi.org/10.7235/hort.2015.14043
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 1:1–18
Gupta S, Agarwal VP, Gupta NK (2012) Efficacy of putrescine and benzyladenine on photosynthesis and productivity in relation to drought tolerance in wheat (Triticum aestivum L.). Physiol Mol Biol Plants 18:331–336
Guruchandran V, Sasikumar C (2013) Effect of polyamines on in vitro organogenesis using shoot tip explants of Stevia rebaudiana Bert. Int J Curr Biotech 1:16–18
Harpaz-Saad S, Yoon GM, Mattoo AK, Kieber JJ (2012) The formation of ACC and competition between polyamines and ethylene for SAM. Ann Plant Rev 44:56
Hasan M, Skalicky M, Jahan MS, Hossain M, Anwar Z, Nie ZF (2021) Spermine: its emerging role in regulating drought stress responses in plants. Cells 10:1–15. https://doi.org/10.3390/cells10020261
Hassan FA, Ali EF, Alamer KH (2018) Exogenous application of polyamines alleviates water stress-induced oxidative stress of Rosa damascena Miller var. Trigintipetala Dieck. S Afr J Bot 116:96–102
Hassan N, Ebeed H, Aljaarany A (2020) Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiol Mol Biol Plants 26:233–245
Hassanein RA, El-Khawas SA, Ibrahim SK, El-Bassiouny HM, Mostafa HA, Abd El-Monem AA (2013) Improving the thermo tolerance of wheat plant by foliar application of arginine or putrescine. Pak J Bot 45:111–118
He L, Nada K, Tachibana S (2002) Effects of spermidine pretreatment through the roots on growth and photosynthesis of chilled cucumber plants (Cucumber sativus L.). J Jpn Soc Hort Sci 71:490–498
He Y, Yang J, Li H, Shao H, Wei C, Wang Y, Li M, Xu C (2015) Exogenous spermine ameliorates high glucose-induced cardiomyocytic apoptosis via decreasing reactive oxygen species accumulation through inhibiting p38/JNK and JAK2 pathways. Int J Clin Exp Pathol 8(12):15537
Hosoya R, Hamana K, Niitsu M, Itoh T (2004) Polyamine analysis for chemotaxonomy of thermophilic eubacteria: polyamine distribution profiles within the orders Aquificales, Thermotogales, Thermodesulfobacteriales, Thermales, Thermoanaerobacteriales, Clostridiales and Bacillales. J Gen Appl Microbiol 50:271–287
Hussain S, Farooq M, Wahid MA, Wahid A (2013) Seed priming with putrescine improves the drought resistance of maize hybrids. Int J Agric Biol 15:1349–1353
Imai A, Akiyama T, Kato T, Sato S, Tabata S, Yamamoto KT, Takahashi T (2004) Spermidine synthase genes are essential for survival of Arabidopsis. Plant Physiol 135:1565–1573. https://doi.org/10.1104/pp.104.041699
Islam MA, Pang JH, Meng FW, Li YW, Ning XU, Chao YANG, Jun LIU (2020) Putrescine, spermidine, and spermine play distinct roles in rice salt tolerance. J Integr Agric 19:643–655
Islam MJ, Uddin MJ, Hossain MA, Henry R, Begum MK, Sohel MAT, Mou MA, Ahn J, Cheong EJ, Lim YS (2022) Exogenous putrescine attenuates the negative impact of drought stress by modulating physio-biochemical traits and gene expression in sugar beet (Beta vulgaris L.). PloS ONE 17(1):0262099
Jarvis BC, Yasmin S, Coleman MT (1985) RNA and protein metabolism during adventitious root formation in stem cuttings of Phaseolus aureus. Physiol Plant 64(1):53–59
Jha UC, Bohra A, Singh NP (2014) Heat stress in crop plants: its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breed 133(6):679–701
Jiang DX, Chu X, Li M, Hou JJ, Tong X, Gao ZP, Chen GX (2020a) Exogenous spermidine enhances salt-stressed rice photosynthetic performance by stabilizing structure and function of chloroplast and thylakoid membranes. Photosynthetica 58:61–71
Jiang X, Zhan J, Wang Q, Wu X, Chen X, Jia B, Liu P, Liu L, Ye Z, Zhu L, Heng W (2020b) Overexpression of the pear PbSPMS gene in Arabidopsis thaliana increases resistance to abiotic stress. Plant Cell Tissue Organ Cult 140:389–401
Jiao Y, Li Z, Xu K, Guo Y, Zhang C, Li T, Jiang Y, Liu G, Xu Y (2017) Study on improving plantlet development and embryo germination rates in in vitro embryo rescue of seedless grapevine. N Z J Crop Hortic Sci 46:39–53. https://doi.org/10.1080/01140671.2017.1338301
Jiménez-Bremont JF, Marina M, Guerrero-Gonzalez MD, Rossi FR, Sánchez-Rangel D, Rodríguez-Kessler M, Ruiz OA, Gárriz A (2014) Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front Plant Sci 5:95
**g J, Guo S, Li Y, Li W (2020) The alleviating effect of exogenous polyamines on heat stress susceptibility of different heat resistant wheat (Triticum aestivum L.) varieties. Sci Rep 10:7467
Joshi K (2022) Reimagining how putrescine functions as a signaling compound: the essential role of synthesis and compartmentation. Doctoral dissertation, Bowling Green State University
Kamiab F, Tavassolian I, Hosseinifarahi M (2020) The role of polyamine in plant science. Biol Fut 71(3):183–194
Kandil MM, El-Saady MB, Mona HM, Afaf MH, Iman ME (2011) Effect of putrescine and uniconazole treatments on flower characters and photosynthetic pigments of Chrysanthemum indicum L. plant. J Am Sci 7:399–408
Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S (2004) Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722
Khan N, Ali S, Tariq H, Latif S, Yasmin H, Mehmood A, Shahid MA (2020) Water conservation and plant survival strategies of Rhizobacteria under drought stress. Agronomy 10(11):1683
Khorshidi M, Hamedi F (2014) Effect of putrescine on lemon balm under salt stress. Int J Agric Crop Sci 7:601–609
Kim KH, Park CS, Park SJ, Kim J, Seo SE, An JE, Ha S, Bae J, Phyo S, Lee J, Kim K (2022) In-situ food spoilage monitoring using a wireless chemical receptor-conjugated graphene electronic nose. Biosens Bioelectron 200:113908
Kotakis C, Theodoropoulou E, Tassis K, Oustamanolakis C, Ioannidis NE, Kotzabasis K (2014) Putrescine, a fast-acting switch for tolerance against osmotic stress. J Plant Physiol 171:48–51
Kusano T, Berberich T, Tateda C, Takahashi Y (2008) Polyamines: essential factors for growth and survival. Planta 228:367–381
Kuznetsov VV, Rakitin VY, Sadomov NG, Dam DV, Stetsenko LA, Shevyakova NI (2002) Do polyamines participate in the long-distance translocation of stress signals in plants? Russ J Plant Physiol 49:120–130
Lasanajak Y, Minocha R, Minocha SC, Goyal R, Fatima T, Handa AK, Mattoo AK (2014) Enhanced flux of substrates into polyamine biosynthesis but not ethylene in tomato fruit engineered with yeast S-adenosylmethionine decarboxylase gene. Amino Acids 46(3):729–742
Laukkanen H, Sarjala T (1997) Effect of exogenous polyamines on Scots pine callus in vitro. J Plant Physiol 150:167–172
Lee S, Baskar TB, Kim JK, Park SU (2016) Enhanced shoot organogenesis in Aloe saponaria following treatment with ethylene inhibitors and polyamines. Biosci Biotech Res Asia 13:17–21
Li C, Pei ZX, Gan LY (2014a) Effects of photoperiod on flowering and polyamine contents of nobile-type dendrobium. Plant Physiol J 50:1167–1170. https://doi.org/10.13592/j.cnki.ppj.2012.0435
Li YR, Cheng KA, Spokas AA, Palmer BJO (2014b) Genetic variation for life history sensitivity to seasonal warming in Arabidopsis thaliana. Genetics 196:569–577
Li Z, Peng Y, Zhang XQ, Ma X, Huang LK, Yan YH (2014c) Exogenous spermidine improves seed germination of white clover under water stress via involvement in starch metabolism, antioxidant defenses and relevant gene expression. Molecules 19:18003–18024
Li Z, Hou J, Zhang Y, Zeng W, Cheng B, Hassan MJ, Zhang Y, Pu Q, Peng Y (2020) Spermine regulates water balance associated with Ca2+-dependent aquaporins (TrTIP2-1, TrTIP2-2, and TrPIP2-7) expression in plants under water stress. Plant Cell Physiol 61:1576–1589
Lin HY, Lin HJ (2019) Polyamines in microalgae: something borrowed, something new. Mar Drugs 17(1):1. https://doi.org/10.3390/md17010001
Liu JH, Moriguchi T (2007) Changes in free polyamine titers and expression of polyamine biosynthetic genes during growth of peach in vitro callus. Plant Cell Rep 26:125–131
Liu JH, Nada K, Honda C, Kitashiba H, Wen XP, Pang XM, Moriguchi T (2006) Polyamine biosynthesis of apple callus under salt stress: importance of the arginine decarboxylase pathway in stress response. J Exp Bot 57:2589–2599. https://doi.org/10.1093/jxb/erl018
Liu Y, Gu D, Wu W, Wen X, Liao Y (2013) The relationship between polyamines and hormones in the regulation of wheat grain filling. PLoS ONE 8:e78196. https://doi.org/10.1371/journal.pone.0078196
Liu JH, Wang W, Wu H, Gong X, Moriguchi T (2015) Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci 6:827
Liu Y, Liang H, Lv X, Liu D, Wen X, Liao Y (2016a) Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol Biochem 100:113–129
Liu Y, Xu H, Wen X, Liao Y (2016b) Effect of polyamine on seed germination of wheat under drought stress is related to changes in hormones and carbohydrates. J Integr Agric 15:2759–2774
Lodeserto P, Rossi M, Blasi P, Farruggia G, Orienti I (2022) Nanospermidine in combination with nanofenretinide induces cell death in neuroblastoma cell lines. Pharmaceutics 14:1215
Lu B, Wang L, Ran X, Tang H, Cao D (2022) Recent advances in fluorescent methods for polyamine detection and the polyamine suppressing strategy in tumor treatment. Biosensors 12:633
Luo J, Liu M, Zhang C, Zhang P, Chen J, Guo Z, Lu S (2017) Transgenic centipedegrass (Eremochloa ophiuroides [Munro] Hack.) overexpression S-adenosylmethionine decarboxylase (SAMDC) gene for improved cold tolerance through involvement of H2O2 and NO signaling. Front Plant Sci 8:1655
Magnes C, Fauland A, Gander E, Narath S, Ratzer M, Eisenberg T, Madeo F, Pieber T, Sinner F (2014) Polyamines in biological samples: rapid and robust quantification by solid-phase extraction online-coupled to liquid chromatography–tandem mass spectrometry. J Chromatogr A 1331:44–51
Mahgoub MH, El-Aziz NG, Mazhar AM (2011) Response of Dahlia pinnata L. plant to foliar spray with putrescine and thiamine on growth, flowering and photosynthetic pigments. Am Eurasian J Agric Environ Sci 10:769–775
Marco F, Alcázar R, Tiburcio AF, Carrasco P (2011) Interactions between polyamines and abiotic stress pathway responses unraveled by transcriptome analysis of polyamine overproducers. OMICS 15:775–781
Marco F, Busó E, Lafuente T, Carrasco P (2019) Spermine confers stress resilience by modulating abscisic acid biosynthesis and stress responses in Arabidopsis plants. Front Plant Sci 10:972
Martínez Pastur G, Arena ME, Benavides MP, Eliasco E, Curvetto N (2007) Role of polyamines during in vitro rhizogenesis of Nothofagus nervosa using successive culture media. New For 34:83–93. https://doi.org/10.1007/s11056-007-9039-6
Mehta RA, Cassol T, Li N, Ali N, Handa AK, Mattoo AK (2002) Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat Biotech 20(6):613–618
Mendoza-de-Gyves E, Royani JI, Rugini E (2007) Efficient method of micropropagation and in vitro rooting of teak (Tectona grandis L.) focusing on large-scale industrial plantations. Ann for Sci 64:73–78. https://doi.org/10.1051/forest:2006090
Meng D, Hou L, Yang S, Meng JJ, Guo F, Li XG (2015) Exogenous polyamines alleviating salt stress on peanuts (Arachis hypogaea) grown in pots. Chin J Plant Ecol 39:1209–1215
Milhinhos A, Prestele J, Bollhöner B, Matos A, Vera-Sirera F, Rambla JL, Ljung K, Carbonell J, Blazquez MA, Tuominen H, Miguel CM (2013) Thermospermine levels are controlled by an auxin-dependent feedback loop mechanism in Populus xylem. The Plant Jour 75(4):685–698
Minocha R, Shortle WC, Long SL, Minocha SC (1994) A rapid and reliable procedure for extraction of cellular polyamines and inorganic ions from plant tissues. J Plant Growth Regul 13:187–193
Mirdehghan SH, Rahemi M, Castillo S, Martínez-Romero D, Serrano M, Valero D (2007) Pre-storage application of polyamines by pressure or immersion improves shelf-life of pomegranate stored at chilling temperature by increasing endogenous polyamine levels. Postharvest Biol Tech 44(1):26–33
Mitsuya Y, Takahashi Y, Berberich T, Miyazaki A, Matsumura H, Takahashi H, Terauchi R, Kusano T (2009) Spermine signaling plays a significant role in the defense response of Arabidopsis thaliana to cucumber mosaic virus. J Plant Physiol 166:626–643
Mostofa MG, Yoshida N, Fujita M (2014) Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul 73:31–44. https://doi.org/10.1007/s10725-013-9865-9
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Mustafavi SH, Shekari F, Abbasi A (2015) Putrescine improve low temperature tolerance of fennel (Foeniculum vulgare Mill.) seeds. Agron Res Mold 48:69–76
Mustafavi SH, Naghdi Badi H, Sękara A, Mehrafarin A, Janda T, Ghorbanpour M, Rafiee H (2018) Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol Plant 40:1–19
Nahar K, Hasanuzzaman M, Rahman A, Alam M, Mahmud JA, Suzuki T, Fujita M (2016a) Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front Plant Sci 7:1104
Nahar K, Rahman M, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016b) Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ Sci Pollut Res 23:21206–21218
Nambeesan SU, Mattoo AK, Handa AK (2019) Nexus between spermidine and floral organ identity and fruit/seed set in tomato. Front Plant Sci 10:1033
Nandy S, Das T, Tudu CK, Mishra T, Ghorai M, Gadekar VS, Anand U, Kumar M, Behl T, Shaikh NK (2022) Unravelling the multi-faceted regulatory role of polyamines in plant biotechnology, transgenics and secondary metabolomics. Appl Microbiol Biotechnol 1:1–25
Niakan M, Rezapour Mahjoob S, Ghorbanli M (2015) Effect of exogenous putrescine on growth, photosynthesis and alkaloid compounds of Datura (Datura stramonium L.) in response to salinity stress under hydroponic conditions. J Sci Technol Greenh Cult 6:111–123. https://doi.org/10.18869/acadpub.ejgcst.6.1.111
Niemi K, Sarjala T, Chen X, Häggman H (2007) Spermidine and the ectomycorrhizal fungus Pisolithus tinctorius synergistically induce maturation of Scots pine embryogenic cultures. J Plant Physiol 164:629–635. https://doi.org/10.1016/j.jplph.2006.03.019
Nikam TD, Ghorpade RP, Nitnaware KM, Ahire ML, Lokhande VH, Chopra A (2013) Micropropagation and non-steroidal anti-inflammatory and anti-arthritic agent boswellic acid production in callus cultures of Boswellia serrata Roxb. Physiol Mol Biol Plants 19:105–116. https://doi.org/10.1007/s12298-012-0137-3
Ouyang H, Hou K, Wang L, Peng W (2017) Optimization protocol for the microwave-assisted extraction of antioxidant components from Pinus elliottii needles using response surface methodology. BioRes 12(1):478–494
Pál M, Szalai G, Janda T (2015) Speculation: polyamines are important in abiotic stress signaling. Plant Sci 237:16–23
Pál M, Tajti J, Szalai G, Peeva V, Végh B, Janda T (2018) Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci Rep 8:1–12
Paul A, Mitter K, Sen RS (2009) Effect of polyamines on in vitro somatic embryogenesis in Momordica charantia L. Plant Cell Tissue Organ Cult 97:303–311. https://doi.org/10.1007/s11240-009-9529-7
Puyang X, An M, Han L, Zhang X (2015) Protective effect of spermidine on salt stress induced oxidative damage in two Kentucky bluegrass (Poa pratensis L.) cultivars. Ecotoxicol Environ Saf 117:96–106
Quinet M, Ndayiragije A, Lefèvre I, Lambillotte B, Dupont-Gillain CC, Lutts S (2010) Putrescine differently influences the effect of salt stress on polyamine metabolism and ethylene synthesis in rice cultivars differing in salt resistance. J Exp Bot 61:2719–2733
Radhakrishnan R, Lee IJ (2013) Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J Plant Growth Regul 32(1):22–30
Rady MM, Hemida KA (2015) Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol Environ Saf 119:178–185
Rajesh MK, Radha E, Sa**i KK, Anitha K (2014) Polyamine-induced somatic embryogenesis and plantlet regeneration in vitro from plumular explants of dwarf cultivars of coconut (Cocos nucifera). Indian J Agric Sci 84(4):527–530
Rakesh B, Sudheer WN, Nagella P (2021) Role of polyamines in plant tissue culture: an overview. Plant Cell Tissue Organ Cult 145:487–506
Rakitin VY, Prudnikova ON, Karyagin VV, Rakitina TY, Vlasov PV, Borisova TA, Novikova GV, Moshkov IE (2008) Ethylene evolution and ABA and polyamine contents in Arabidopsis thaliana during UV-B stress. Russian J Plant Physiol 55(3):321–327
Rebecca LJ, Das S, Dhanalakshmi V, Anbuselvi S (2010) Effect of exogenous spermidine on salinity tolerance with respect to seed germination. Int J Appl Agric Res 5:163–169
Reis RS, de Moura VE, Heringer AS, Santa-Catarina C, Silveira V (2016) Putrescine induces somatic embryo development and proteomic changes in embryogenic callus of sugarcane. J Proteomics 130:170–179. https://doi.org/10.1016/j.jprot.2015.09.029
Rihan HZ, Al-Issawi M, Fuller MP (2017) Advances in physiological and molecular aspects of plant cold tolerance. J Plant Interact 12:143–157
Romanov GA, Rakova NY, Vanyushin BF (2004) Polyamines suppress expression of the cytokinin-dependent transgene in Arabidopsis. Doklady Biochemistry and Biophysics, vol 398. Kluwer Academic Publishers-Plenum Publishers, Amsterdam, pp 288–290
Romero FM, Maiale SJ, Rossi FR, Marina M, Ruíz OA, Gárriz A (2018) Polyamine metabolism responses to biotic and abiotic stress. Methods Mol Biol 1694:37–49
Roy M, Wu R (2002) Overexpression of S-adenosylmethionine decarboxylase gene in rice increases polyamine level and enhances sodium chlorides-stress tolerance. Plant Sci 163:987–992
Roy P, Niyogi K, Sengupta DN, Ghosh B (2005) Spermidine treatment to rice seedlings recovers salinity stress-induced damage of plasma membrane and PM-bound H+-ATPase in salt-tolerant and salt-sensitive rice cultivars. Plant Sci 168:583–591
Sagor GH, Berberich T, Takahashi Y, Niitsu M, Kusano T (2013) The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic Res 22:595–605
Sathish D, Theboral J, Vasudevan V, Pavan G, Ajithan C, Appunu C, Manickavasagam M (2020a) Exogenous polyamines enhance somatic embryogenesis and Agrobacterium tumefaciens-mediated transformation efficiency in sugarcane (Saccharum spp. hybrid). In Vitro Cell Dev Biol Plant 56:29–40. https://doi.org/10.1007/s11627-019-10022-6
Sathish D, Vasudevan V, Theboral J, Elayaraja D, Appunu C, Siva R, Manickavasagam M (2020b) Efficient direct plant regeneration from immature leaf roll explants of sugarcane (Saccharum officinarum L.) using polyamines and assessment of genetic fidelity by SCoT markers. In Vitro Cell Dev Biol Plant 54:399–412. https://doi.org/10.1007/s11627-018-9910-5
Satish L, Rency AS, Rathinapriya P, Ceasar SA, Pandian S, Rameshkumar R, Rao TB, Balachandran SM, Ramesh M (2016) Influence of plant growth regulators and spermidine on somatic embryogenesis and plant regeneration in four Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn). Plant Cell Tissue Organ Cult 124:15–31. https://doi.org/10.1007/s11240-015-0870-8
Sauter M, Moffatt B, Saechao MC, Hell R, Wirtz M (2013) Methionine salvage and S-adenosylmethionine: essential links between sulfur, ethylene and polyamine biosynthesis. Biochem J 451(2):145–154
Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, Dindaroglu T, Abdul-Wajid HH, Battaglia ML (2021) Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10(2):259
Setia N, Setia RC (2018) Polyamines: an overview and prospects in crop improvement. Crop Improv Strateg App 376–393
Shah AA, Riaz L, Siddiqui MH, Nazar R, Ahmed S, Yasin NA, Ali A, Mukherjee S, Hussaan M, Javad S (2022) Spermine-mediated polyamine metabolism enhances arsenic-stress tolerance in Phaseolus vulgaris by expression of zinc-finger proteins related genes and modulation of mineral nutrient homeostasis and antioxidative system. Environ Pollut 300:118941
Shi J, Fu XZ, Peng T, Huang XS, Fan QJ, Liu JH (2010) Spermine pre-treatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol 30:914–922
Shi H, Ye T, Chang Z (2013) Comparative proteomic and physiological analyses reveal the protective effect of exogenous polyamines in the Bermuda grass (Cynodon dactylon). Response to salt and drought stresses. J Proteome Res 12:4951–4964
Shiozaki S, Ogata T, Horiuchi S, Zhuo X (1998) Involvement of polyamines in gibberellin-induced development of seedless grape berries. Plant Growth Regul 25:187–193. https://doi.org/10.1023/A:1006043116190
Sivanandhan G, Mariashibu TS, Arun M, Rajesh M, Kasthurirengan S, Selvaraj N, Ganapathi A (2011) The effect of polyamines on the efficiency of multiplication and rooting of Withania somnifera (L.) Dunal and content of some withanolides in obtained plants. Acta Physiol Plant 33:2279–2288. https://doi.org/10.1007/s11738-011-0768-y
Sobieszczuk-Nowicka E (2017) Polyamine catabolism adds fuel to leaf senescence. Amino Acids 49(1):49–56
Song Y, Diao Q, Qi H (2014) Putrescine enhances chilling tolerance of tomato (Lycopersicon esculentum Mill.) through modulating antioxidant systems. Acta Physiol Plant 36:3013–3027
Sun X, Wang Y, Sui N (2018a) Transcriptional regulation of bHLH during plant response to stress. Biochem Biophy Res Commu 503(2):397–401
Sun X, Wang Y, Tan J, Al E (2018b) Effects of exogenous putrescine and D-Arg on physiological and biochemical indices of anthurium under chilling stress. Jiangsu J Agric Sci 34:152–157. https://doi.org/10.3969/j.issn.1000-4440.2018.01.022
Sundararajan S, Sivakumar HP, Nayeem S, Rajendran V, Subiramani S, Ramalingam S (2021) Influence of exogenous polyamines on somatic embryogenesis and regeneration of fresh and long-term cultures of three elite indica rice cultivars. Cereal Res Commun 49:245–253. https://doi.org/10.1007/s42976-020-00098-x
Takahashi Y, Tahara M, Yamada Y, Al E (2017) Characterization of the polyamine biosynthetic pathways and salt stress response in Brachypodium distachyon. J Plant Growth Reg 37:625–634
Takano A, Kakehi JI, Takahashi T (2012) Thermospermine is not a minor polyamine in the plant kingdom. Plant Cell Physiol 53:606–616. https://doi.org/10.1093/pcp/pcs019
Talaat IM, Balbaa LK (2010) Physiological response of sweet basil plants (Ocimum basilicum L.) to putrescine and trans-cinnamic acid. Am Eurasian J Agric Environ Sci 8:438–445
Talaat IM, Bekheta MA, Mahgoub MH (2005) Physiological response of periwinkle plants (Catharanthus roseus L.) to tryptophan and putrescine. Int J Agric Biol 7:210–213
Tan M, Hassan MJ, Peng Y, Feng G, Huang L, Liu L, Liu W, Han L, Li Z (2022) Polyamines metabolism interacts with γ-aminobutyric acid, proline and nitrogen metabolisms to affect drought tolerance of cree** bentgrass. Int J Mol Sci 23(5):2779
Tang W, Newton RJ, Outhavong V (2004) Exogenously added polyamines recover browning tissues into normal callus cultures and improve plant regeneration in pine. Physiol Plant 122:386–395. https://doi.org/10.1111/j.1399-3054.2004.00406.x
Tatte SU, Singh A, Ahlawat TR (2015) Effect of polyamines on postharvest quality and vaselife of rose var. Samurai. Bioscan 10:675–678
Thiruvengadam M, Rekha KT, Kim EH, Praveen N, Chung IM (2013) Effect of exogenous polyamines enhances somatic embryogenesis via suspension cultures of spine gourd (Momordica dioica Roxb. Ex. Willd). Australian J Crop Sci 7:446–453
Tian J, Wang LP, Yang YJ, Sun J, Guo SR (2012) Exogenous spermidine alleviates the oxidative damage in cucumber seedlings subjected to high temperatures. J Am Soc Hortic Sci 137(1):11–19
Todorova D, Katerova Z, Alexieva V, Sergiev I (2015) Polyamines—possibilities for application to increase plant tolerance and adaptation capacity to stress. Gen Plant Physiol 5:123–144
Tomar PC, Lakra N, Mishra SN (2013) Cadaverine: a lysine catabolite involved in plant growth and development. Plant Signal Behav 8(10):e25850. https://doi.org/10.4161/psb.25850
Tsaniklidis G, Pappi P, Tsafouros A, Charova SN, Nikoloudakis N, Roussos PA, Paschalidis KA, Delis C (2020) Polyamine homeostasis in tomato biotic/abiotic stress cross-tolerance. Gene 727:144230
Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh EI, Scherer GF (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47:346–354
Uehara Y, Takahashi Y, Berberich T, Miyazak A, Takahashi H, Matsui K, Ohme-Takagi T, Satoh H, Terauchi R, Kusano T (2005) Tobacco ZFT1, a transcriptional repressor with a Cys2/His2 type zinc finger motif that functions in spermine signaling pathway. Plant Mol Biol 59(3):435–448
Urano K, Yoshiba Y, Nanjo T, Ito T, Yamaguchi-Shinozaki K, Shinozaki K (2004) Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem Biophys Res Commun 313:369–375
Vasudevan V, Subramanyam K, Elayaraja D, Karthik S, Vasudevan A, Manickavasagam M (2017) Assessment of the efficacy of amino acids and polyamines on regeneration of watermelon (Citrullus lanatus Thunb.) and analysis of genetic fidelity of regenerated plants by SCoT and RAPD markers. Plant Cell Tissue Organ Cult 130:681–687. https://doi.org/10.1007/s11240-017-1243-2
Venkatachalam L, Bhagyalakshmi N (2008) Spermine-induced morphogenesis and effect of partial immersion system on the shoot cultures of banana. Appl Biochem Biotechnol 151:502–511. https://doi.org/10.1007/s12010-008-8226-z
Viu AF, Viu MA, Tavares AR, Vianello F, Lima GP (2009) Endogenous and exogenous polyamines in the organogenesis in Curcuma longa L. Sci Hortic 121:501–504. https://doi.org/10.1016/j.scienta.2009.03.003
Vuosku J, Karppinen K, Muilu-Mäkelä R, Kusano T, Sagor GH, Avia K, Alakärppä E, Kestilä J, Suokas M, Nickolov K, Hamberg L (2018) Scots pine aminopropyltransferases shed new light on evolution of the polyamine biosynthesis pathway in seed plants. Ann Bot 121:1243–1256
Walker MA, Roberts DR, Dumbroff EB (1988) Effects of cytokinin and light on polyamines during the greening response of cucumber cotyledons. Plant Cell Physiol 29:201–205
Wang Q, Yin X (2014) Alleviative effects of different kinds of exogenous polyamines on salt injury of soybean seedlings. J Henan Agric Sci 43(4):48–55
Wang X, Ying W, Dunlap K, Lin G, Satterfield MC, Burghardt RC, Wu G, Bazer FW (2014) Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod 90:84–91
Wang W, Paschalidis K, Feng JC, Song J, Liu JH (2019) Polyamine catabolism in plants: a universal process with diverse functions. Front Plant Sci 10:561
Wimalasekera R, Tebartz F, Scherer GF (2011) Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci 181(5):593–603
Wolkovich EM, Cook BI, Allen JM, Crimmins TM, Betancourt JL, Travers SE, Pau S, Regetz J, Davies TJ, Kraft NJ, Ault TR, Bolmgren K, Mazer SJ, McCabe GJ, McGill BJ, Parmesan C, Salamin N, Schwartz MD, Cleland EE (2012) Warming experiments under predict plant phenological responses to climate change. Nature 485:494–497
Wortham BW, Oliveira MA, Patel CN (2007) Polyamines in bacteria: pleiotropic effects yet specific mechanisms. In: Perry RD, Fetherston JD (eds) The genus Yersinia. Springer, Berlin, pp 106–115
Wrede F (1925) Überdie aus dem menschlichen Sperma isolierte Base Spermin. Deutsche Medizinische Wochenschrift 51:24
**ong F, Liao J, Ma Y, Wang Y, Fang W, Zhu X (2018) The protective effect of exogenous putrescine in the response of tea plants (Camellia sinensis) to salt stress. HortSci 53:1640–1646
**ong W, Wang Y, Guo Y, Tang W, Zhao Y, Yang G, Pei Y, Chen J, Song X, Sun J (2022) Transcriptional and metabolic responses of maize shoots to long-term potassium deficiency. Front Plant Sci 13:922581
Xu L (2015) The effect of polyamineon flower bud differentiation and bud germination of chrysanthemum. Shandong Agric Univ 2:31–36
Xu C, Wu X (2009) Impact of DArg on drought resistance and endogenous polyamines in mycorrhizal Pinus massoniana. J Nan**g Univ 52:19
Xu S, Hu J, Li Y, Ma W, Zheng Y, Zhu S (2011) Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regul 63:279–290. https://doi.org/10.1007/s10725-010-9528-z
Xu L, **ng ST, Sun X (2014) Effects of polyamines on hormones contents and the relationship with the flower bud differentiation in chrysanthemum. Plant Physiol J 50:1195–1202. https://doi.org/10.13592/j.cnki.ppj.2014.0212
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Miyazaki A, Takahashi T, Michael A, Kusano T (2006) The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett 580:6783–6788
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Takahashi T, Michael AJ, Kusano TA (2007) Protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem Biophy Res Commun 352:486–490
Yamazaki S, Iwama A, Takayanagi SI, Morita Y, Eto K, Ema H, Nakauchi H (2006) Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J 25:3515–3523
Yu L, Geng S, Zhao-Hui Z, Xue-Lian Z, Ke-Jun D (2017) Overexpression of SAMDC gene from Salvia miltiorrhiza enhances drought tolerance in transgenic tobacco (Nicotiana tabacum). J Agric Biotech 25:729–738
Yu Z, Jia D, Liu T (2019) Polyamine oxidases play various roles in plant development and abiotic stress tolerance. Plants 8(6):184
Zeid IM, Shedeed ZA (2006) Response of alfalfa to putrescine treatment under drought stress. Biol Plant 50(4):635–640
Zhang RH, Li J, Guo SR, Tezuka T (2009) Effects of exogenous putrescine on gas-exchange characteristics and chlorophyll fluorescence of NaCl-stressed cucumber seedlings. Photosynth Res 100:155–162
Zhang N, Shi X, Guan Z, Zhao S, Zhang F, Chen S, Fang W, Chen F (2016) Treatment with spermidine protects chrysanthemum seedlings against salinity stress damage. Plant Physiol Biochem 105:260–270
Zhu X, Wang L, Yang R, Han Y, Hao J, Liu C, Fan S (2019) Effects of exogenous putrescine on the ultrastructure of and calcium ion flow rate in lettuce leaf epidermal cells under drought stress. Hort Environ Biotech 60:479–490
Zhu H, Tian W, Zhu X, Tang X, Wu L, Hu X, ** S (2020) Ectopic expression of GhSAMDC1 improved plant vegetative growth and early flowering through conversion of spermidine to spermine in tobacco. Sci Rep 10(1):1–1
Acknowledgements
Authors are grateful to Department of Biotechnology, Dr Y S P University of Horticulture and Forestry, Solan, India, National Agri-Food Biotechnology Institute, Mohali, India and Guru Jambheshwar University of Science & Technology, Haryana, India for providing the infrastructure and support for the study.
Author information
Authors and Affiliations
Contributions
Conceptualization: VS and MT, Search strategy, article outline, tables and figures: VS, AJ, and SC, Writing of rough draft: AJ, SC, NV, and HS. Article editing: VS, MT, ST, and VC. Final review: corresponding author. Before submitting the manuscript, a final revision and approval of manuscript has been performed by all the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: M. Naeem.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jangra, A., Chaturvedi, S., Kumar, N. et al. Polyamines: The Gleam of Next-Generation Plant Growth Regulators for Growth, Development, Stress Mitigation, and Hormonal Crosstalk in Plants—A Systematic Review. J Plant Growth Regul 42, 5167–5191 (2023). https://doi.org/10.1007/s00344-022-10846-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10846-4