Abstract
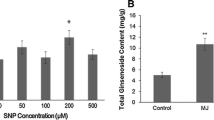
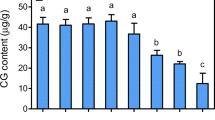
The adventitious root (AR) culture of Panax ginseng C.A. Meyer is an alternative route for mass production of ginsenosides. During the AR culture of P. ginseng, methyl jasmonate (MeJA) is frequently used as an abiotic elicitor for improving the ginsenoside production. However, knowledge on the signaling molecules involved in MeJA-induced ginsenoside synthesis is lacking to date. Therefore, the present study used MeJA to treat 30-day-old bioreactor-cultured ARs and determined the changes of ginsenosides and three signaling molecules, including putrescine (Put), nitric oxide (NO), and hydrogen peroxide (H2O2). Meanwhile, the effects of Put [(d-(-)-arginine, (d-Arg)] and nitric oxide synthase (NOS) [NG-nitro-l-arginine methyl ester (l-NAME) and S,S′-1,3-phenylenebis (1,2-ethanediyl)-bis-isothiourea (PBITU)] inhibitors and the H2O2 [catalase (CAT)] scavenger on the three signaling molecules and ginsenoside production in ARs were investigated. Results showed that MeJA promoted ginsenoside accumulation, and the maximum content of total ginsenosides was reached after 8 days of MeJA elicitation. The Put, NO, and H2O2 production increased with MeJA treatment. The Put and NO contents peaked at 12 h, whereas the H2O2 content peaked at 24 h. No obvious change (p < 0.05) was found in the MeJA-untreated control group, demonstrating that the change pattern of Put, NO, and H2O2 contents was the specificity of MeJA elicitation. In MeJA-stimulated ARs, Put and ginsenoside contents were significantly decreased with d-Arg treatment. The NOS activity and NO content and ginsenoside contents were also decreased with l-NAME and PBITU treatments. The H2O2 and ginsenoside contents were decreased with CAT treatment. These results demonstrated the enhancement effects of MeJA on the production of Put, NO, H2O2, and ginsenosides, but the increase of ginsenosides was eliminated by the inhibitor or scavenger of the signaling molecules, which proved that Put, NO, and H2O2 involved in MeJA-induced ginsenoside synthesis of P. ginseng ARs.








Similar content being viewed by others
References
Ali MB, Yu KW, Hahn EJ, Paek KY (2006) Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep 25:613–620
Bae KH, Choi YE, Shin CG, Kim YY, Kim YS (2006) Enhanced ginsenoside productivity by combination of ethephon and methyl jasmoante in ginseng (Panax ginseng C.A. Meyer) adventitious root cultures. Biotechnol Lett 28:1163–1166
Baque MA, Murthy HN, Paek KY (2014) Adventitious root culture of Morinda citrifolia in bioreactors for production of bioactive compounds. In: Paek KY, Murthy HN, Zhong JJ (eds) Production of biomass and bioactive compounds using bioreactor technology. Springer, Dordrecht, pp 185–222
Cacho M, Domínguea A, Elena-Rosselló J (2013) Role of polyamines in regulating silymarin production in Silybum marianum (L.) Gaertn (Asteraceae) cell cultures under conditions of calcium deficiency. J Plant Physiol 170:1344–1348
Chen LR, Chen YJ, Lee CY, Lin TY (2007) MeJA-induced transcriptional changes in adventitious roots of Bupleurum kaoi. Plant Sci 173:12–24
Cheng HY, Song SQ (2005) Advances in nitric oxide biology of plants. Chin Bull Bot 22:723–737
Choi KT (2008) Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C.A. Meyer Acta Pharm Sin 29:1109–1118
Durner J, Wendehenne D, Klessig D (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA 95:10328–10333
Fariduddin Q, Varshney P, Yusuf M, Ahmad A (2013) Polyamines: potent modulators of plant responses to stress. J Plant Interact 8:1–16
Han L, Piao XC, Jiang J, Jiang X, Lian L (2019) A high production of flavonoids and anthraquinones via adventitious root culture of Oplopanax elatus and evaluating antioxidant activity. Plant Cell Tiss Organ Cult 137:173–179
Hao WF, Guo HB, Zhang J, Hu G, Yao Y, Dong J (2014) Hydrogen peroxide is involved in salicylic acid-elicited rosmarinic acid production in Salvia miltiorrhiza cell cultures. Sci World J 2014:843764
Hu XY, Neill SJ, Cai WM, Tang ZC (2003a) Hydrogen peroxide and jasmonic acid mediate oligogalacturonic acid-induced saponin accumulation in suspension-cultured cells of Panax ginseng. Physiol Plantarum 118:414–421
Hu XY, Neill SJ, Cai WM, Tang ZC (2003b) Nitric oxide mediates elicitor-induced saponin synthesis in cell cultures of Panax ginseng. Funct Plant Biol 30:901–907
Huber JL, Huber SC, Campbell WH, Redinbaugh MG (1992) Reversible light/dark modulation of spinach leaf nitrate reductase activity involves protein phosphorylation. Arch Bioche Biophys 296:58–65
Jabs T, Tschope M, Colling C, Hahlbrock K, Scheel D (1997) Elicitor-stimulated ion fluxes and O2- from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci 94:4800–4805
Jian WW, Jian YW (2005) Involvement of nitric oxide in elicitor-induced defense responses and secondary metabolism of Taxus chinensis cells. Nitric Oxide Biol Chem 11:298–306
Kusano T, Yamaguchi K, Berberich T, Takahashi Y (2007) Advances in polyamine research. J Plant Res 120:345–350
Le KC, Im WT, Paek KY, Park SY (2018) Biotic elicitation of ginsenoside metabolism of mutant adventitious root culture in Panax ginseng. Appl Microbiol Biotechnol 102:1687–1697
Li JX, Liu SJ, Wang J, Li J, Liu DH, Li JL, Gao WY (2016) Fungal elicitors enhance ginsenosides biosynthesis, expression of functional genes as well as signal molecules accumulation in adventitious roots of Panax ginseng C. A Mey J Biotechnol 239:106–114
Liu J, Ji XJ, Liu YL (2002) High performance liquid chromatography method for measuring polyamine content in plant tissue. Plant Physiol Commun 38:596–598
Liu YT, Wang XD, Zhou WY, Zhan YG, Fan GZ (2014) Polyamine-mediated triterpenoid synthesis in suspension cells of Betula platyphylla induced by fungal elicitor. Chin Trad Herbal Drugs 45:695–700
Modolo LV, Cunha FQ, Braga MR, Salgado I (2002) Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorum f. sp. meridionalis elicitor. Plant Physiol 130:1288–1297
Murashige T, Skoog F (1963) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395
Rahimi S, Devi BSR, Khorolragchaa A, Kim YJ, Kim JH, Jung SK, Yang DC (2014) Effect of salicylic acid and yeast extract on the accumulation of jasmonic acid and sesquiterpenoids in Panax ginseng adventitious roots. Russ J Plant Physiol 61:811–817
Sharma M, Gupta SK, Deeba F, Pandey V (2018) Effects of reactive oxygen species on crop productivity: an overview. In: Singh VP, Singh S, Tripathi DK, Prasad SM, Chauhan DK (eds) Revisiting the role of reactive oxygen species (ROS) in plants: ROS boon or bane for plants?. Wiley, Cochran, pp 117–135
Shi HT, Chan ZL (2014) Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J Integr Plant Biol 56:114–121
Tang M, Smith CJ (2001) Elicitor induced defence responses in Medicago sativa. New Phytol 149:401–418
Tarchevsky IA (2000) Elicitor-induced signaling pathways and their interaction. Russ J Plant Physiol 47:285–294
Thanh NT, Murthy HN, Yu KW, Hahn EJ, Paek KY (2005) Methyl jasmonate elicitation enhanced synthesis of ginsenoside by cell suspension cultures of Panax ginseng in 5 L balloon type bubble bioreactors. Appl Microbiol Biotechnol 67:197–201
Walden R, Cordeiro A, Tiburcio AF (1997) Polyamines: small molecules triggering pathways in plant growth and development. Plant Physiol 113:1009–1013
Wang J, Gao WY, Zuo BM, Zhang LM, Huang LQ (2013) Effect of methyl jasmonate on the ginsenoside content of Panax ginseng adventitious root cultures and on the genes involved in triterpene biosynthesis. Res Chem Intermediat 39:1973–1980
Wi SJ, Park KY (2002) Antisense expression of carnation cDNA encoding ACC synthase or ACC oxidase enhances polyamine content and abiotic stress tolerance in transgenic tobacco plants. Mol Cells 13:209–220
Xu MJ (2007) Nitric oxide: a potential key point of the signaling network leading to plant secondary metabolite biosynthesis. Prog Nat Sci 17:1397–1404
Xu MJ, Dong JF (2005) O2–000 from elicitor-induced oxidative burst is necessary for triggering phenylalanine ammonia-lyase activation and catharanthine synthesis in Catharanthus roseus cell cultures. Enzyme Microb Technol 36:280–284
Xu MJ, Dong JF, Zhu M (2004) Involvement of NO in elicitor-induced PAL activation and taxol synthesis in Taxus chinensis suspension cells. Chin Sci Bull 49:1038–1043
Xu MJ, Dong JF, Zhu MY (2005) Nitric oxide mediates the fungal elicitor-induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic-acid-dependent signal pathway. Plant Physiol 139:991–998
Yoshimatsu K (2008) Tissue culture of medicinal plants: micropropagation, transformation and production of useful secondary metabolites. Stud Nat Prod Chem 34:647–752
Yu Y, Zhang WB, Li XY, Piao XC, Jiang J, Lian ML (2016) Pathogenic fungal elicitors enhance ginsenoside biosynthesis of adventitious roots in Panax quinquefolius during bioreactor culture. Ind Crop Prod 94:729–735
Zhao J, Zheng SH, Fujuta K, Sakai K (2004) Jasmonate and ethylene signalling and their interaction are integral parts of the elicitor signalling pathway leading to β-thujaplicin biosynthesis in Cupressus lusitanica cell cultures. J Exp Bot 55:1003–1012
Zhou B, Guo Z, **ng J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Acknowledgements
The financial support of this work was provided by the National Natural Science Foundation of China (81960685) and the Jilin Scientific and Technological Development Program (20180101278JC).
Author information
Authors and Affiliations
Contributions
X-HW, M-ZF designed the methodology; X-FL performed the investigation; X-CP performed the data analysis; RG participated in writing and original draft preparation; M-LL participated in writing, review/editing and supervision.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, XH., Fan, MZ., Li, XF. et al. Involvement of Putrescine, Nitric Oxide, and Hydrogen Peroxide in Methyl Jasmonate-Induced Ginsenoside Synthesis in Adventitious Root Cultures of Panax ginseng C.A. Meyer. J Plant Growth Regul 40, 1440–1449 (2021). https://doi.org/10.1007/s00344-020-10199-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10199-w