Abstract
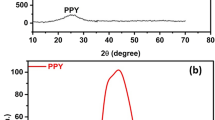
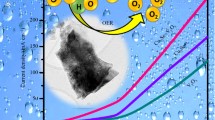
Electrochemical water splitting stands as a promising method for harnessing energy from renewable sources. However, substantial overpotential required for sluggish oxygen evolution reaction (OER) hampers its widespread adoption. In this study, a CuSe@PPy hybrid is being created by hydrothermally layering polypyrrole on top of CuSe. This hybrid electrocatalyst outperforms both pure CuSe and PPy in terms of OER efficiency. Structural and morphological analyses, including powder X-ray diffraction, scanning electron microscopy, Fourier transform infrared spectroscopy, and Brunauer–Emmett–Teller (BET), confirm that the synthesized CuSe@PPy composite exhibits high crystallinity, nanostructured granular morphology, and a hexagonal structure with a large surface area. Evaluation of its electrocatalytic performance for water oxidation in a 1 M KOH alkaline medium reveals CuSe@PPy hybrid's exceptional durability, achieving 35 mA cm−2 for 100 h. This durability is attributed to PPy coating on its surface, which facilitates efficient electron conduction. Coupling of PPy with CuSe leads to reduced overpotential (248 mV), a lower Tafel slope (30 mV/dec), and decreased charge transfer resistance (2.16 Ω), enhancing OER efficiency. By modifying surface of CuSe with a conducting polymer like PPy, this study underscores potential for improving performance in various applications, including photoelectron-catalytic research and stabilizing material activity.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
C. Wei, Z.J. Xu, The comprehensive understanding of as an evaluation parameter for electrochemical water splitting. Small Methods. 2, 1800168 (2018)
L. Li, P. Wang, Q. Shao, X.J. Huang, Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 49, 3072–3106 (2020)
J. Wang, W. Cui, Q. Liu, Z. **ng, A.M. Asiri, X.J. Sun, Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 28, 215–230 (2016)
B. Zhang, Y. Zheng, T. Ma, C. Yang, Y. Peng, Z. Zhou, M. Zhou, S. Li, Y. Wang, C.J. Cheng, Designing MOF nanoarchitectures for electrochemical water splitting. Adv. Mater. 33, 2006042 (2021)
Z.P. Ifkovits, J.M. Evans, M.C. Meier, K.M. Papadantonakis, N.S. Lewis, Decoupled electrochemical water-splitting systems: a review and perspective. Energy Environ. Sci. 14, 4740–4759 (2021)
J. Joo, T. Kim, J. Lee, S.I. Choi, K.J. Lee, Morphology-controlled metal sulfides and phosphides for electrochemical water splitting. Adv. Mater. 31, 1806682 (2019)
C. Zhu, Q. Shi, S. Feng, D. Du, Y.J. Lin, Single-atom catalysts for electrochemical water splitting. ACS Energy Lett. 3, 1713–1721 (2018)
Y. Tan, H. Wang, P. Liu, Y. Shen, C. Cheng, A. Hirata, T. Fujita, Z. Tang, M.J.E. Chen, E. Science, Versatile nanoporous bimetallic phosphides towards electrochemical water splitting. Energy Environ. Sci. 9, 2257–2261 (2016)
H. Zhang, A.W. Maijenburg, X. Li, S.L. Schweizer, R.B.J. Wehrspohn, Bifunctional heterostructured transition metal phosphides for efficient electrochemical water splitting. Adv. Funct. Mater.Funct. Mater. 30, 2003261 (2020)
Q. Hu, G. Li, Z. Han, Z. Wang, X. Huang, H. Yang, Q. Zhang, J. Liu, C. He, Recent progress in the hybrids of transition metals/carbon for electrochemical water splitting. J. Mater. Chem. 7, 14380–14390 (2019)
M. Hassan, Y. Slimani, M.A. Gondal, M.J. Mohamed, S. Güner, M.A. Almessiere, A.M. Surrati, A. Baykal, S. Trukhanov, A. Trukhanov, Structural parameters, energy states and magnetic properties of the novel Se-doped NiFe2O4 ferrites as highly efficient electrocatalysts for HER. Ceram. Int. 48, 24866–24876 (2022)
S. Trukhanov, A. Trukhanov, V. Turchenko, A.V. Trukhanov, E. Trukhanova, D. Tishkevich, V. Ivanov, T. Zubar, M. Salem, V. Kostishyn, Polarization origin and iron positions in indium doped barium hexaferrites. Ceram. Int. 44, 290–300 (2018)
D. Vinnik, V. Kokovkin, V. Volchek, V. Zhivulin, P. Abramov, N. Cherkasova, Z. Sun, M. Sayyed, D. Tishkevich, A. Trukhanov, Electrocatalytic activity of various hexagonal ferrites in OER process. Mater. Chem. Phys. 270, 124818 (2021)
M. Zdorovets, A. Kozlovskiy, D. Tishkevich, T. Zubar, A. Trukhanov, The effect of do** of TiO2 thin films with low-energy O2+ ions on increasing the efficiency of hydrogen evolution in photocatalytic reactions of water splitting. J. Mater. Sci. Mater. Electron. 31, 21142–21153 (2020)
W. Li, C. Wang, X. Lu, Integrated transition metal and compounds with carbon nanomaterials for electrochemical water splitting. J. Mater. Chem. A. 9, 3786–3827 (2021)
M.-S. Balogun, Y. Huang, W. Qiu, H. Yang, H. Ji, Y.J.M.T. Tong, Updates on the development of nanostructured transition metal nitrides for electrochemical energy storage and water splitting. Mater. Today 20, 425–451 (2017)
J. Wang, X. Yue, Y. Yang, S. Sirisomboonchai, P. Wang, X. Ma, A. Abudula, G. Guan, Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: a review. J. Alloy. Compd. 819, 153346 (2020)
A. Trukhanov, V. Turchenko, I. Bobrikov, S. Trukhanov, I. Kazakevich, A. Balagurov, Crystal structure and magnetic properties of the BaFe12− xAlxO19 (x = 0.1–1.2) solid solutions. J. Magn. Magn. Mater.Magn. Magn. Mater. 393, 253–259 (2015)
A. Trukhanov, V. Kostishyn, L. Panina, V. Korovushkin, V. Turchenko, P. Thakur, A. Thakur, Y. Yang, D. Vinnik, E. Yakovenko, Control of electromagnetic properties in substituted M-type hexagonal ferrites. J. Alloy. Compd. 754, 247–256 (2018)
M.A. Almessiere, A.V. Trukhanov, Y. Slimani, K. You, S.V. Trukhanov, E.L. Trukhanova, F. Esa, A. Sadaqat, K. Chaudhary, M. Zdorovets, Correlation between composition and electrodynamics properties in nanocomposites based on hard/soft ferrimagnetics with strong exchange coupling. Nanomaterials 9, 202 (2019)
S. Li, E. Li, X. An, X. Hao, Z. Jiang, G. Guan, Transition-metal-based catalysts for electrochemical water splitting at high current density: current status and perspectives. Nanoscale 13, 12788 (2021)
J. Ying, H. Wang, Strategies for develo** transition metal phosphides in electrochemical water splitting. Front. Chem. 9, 700020 (2021)
R. Jamil, R. Ali, S. Loomba, J. **an, M. Yousaf, K. Khan, B. Shabbir, C.F. McConville, A. Mahmood, N. Mahmood, The role of nitrogen in transition-metal nitrides in electrochemical water splitting. Chem. Catal. 1, 802–854 (2021)
R. Gao, J. Zhu, D. Yan, Transition metal-based layered double hydroxides for photo (electro) chemical water splitting: a mini review. Nanoscale 13, 13593 (2021)
X. Chen, X. Wang, X. Zhang, D. Liu, K. Srinivas, F. Ma, B. Wang, B. Yu, Q. Wu, Y. Chen, Facile and scalable synthesis of heterostructural NiSe2/FeSe2 nanoparticles as efficient and stable binder-free electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 46, 35198–35208 (2021)
K. Wang, Z. Lin, Y. Tang, Z. Tang, C.-L. Tao, D.-D. Qin, Y. Tian, Selenide/sulfide heterostructured NiCo2Se4/NiCoS4 for oxygen evolution reaction, hydrogen evolution reaction, water splitting and Zn-air batteries. Electrochim. Acta 368, 137584 (2021)
X. Peng, Y. Yan, X. **, C. Huang, W. **, B. Gao, P.K. Chu, Recent advance and prospectives of electrocatalysts based on transition metal selenides for efficient water splitting. Nano Energy 78, 105234 (2020)
X. **a, L. Wang, N. Sui, V.L. Colvin, W.Y. William, Recent progress in transition metal selenide electrocatalysts for water splitting. Nanoscale 12, 12249–12262 (2020)
X. Du, G. Ma, X. Zhang, Experimental and theoretical understanding on electrochemical activation processes of nickel selenide for excellent water-splitting performance: comparing the electrochemical performances with M-NiSe (M=Co, Cu, and V). ACS Sustain. Chem. Eng. 7, 19257–19267 (2019)
M. Das, A. Biswas, Z.B. Khan, R.S. Dey, Tuning the electronic structure of cobalt selenide on copper foam by introducing a Ni buffer layer for highly efficient electrochemical water splitting. Inorganic Chem. 61, 13218–13225 (2022)
S.S. Jayaseelan, N. Bhuvanendran, Q. Xu, H. Su, Co3O4 nanoparticles decorated polypyrrole/carbon nanocomposite as efficient bi-functional electrocatalyst for electrochemical water splitting. Int. J. Hydrogen Energy 45, 4587–4595 (2020)
L. Naderi, S. Shahrokhian, Cobalt vanadium chalcogenide microspheres decorated with dendrite-like fiber nanostructure for flexible and wire-typed energy conversion and storage micro-devices. Nanoscale 14, 9150 (2022)
K. Brijesh, K. Bindu, D. Shanbhag, H.S. Nagaraja, Chemically prepared Polypyrrole/ZnWO4 nanocomposite electrodes for electrocatalytic water splitting. Int. J. Hydrogen Energy 44, 757–767 (2019)
S. Ghosh, S.R. Keshri, S. Bera, R.N. Basu, Enhanced solar hydrogen generation using Cu–Cu2O integrated polypyrrole nanofibers as heterostructured catalysts. Int. J. Hydrogen Energy 45, 6159–6173 (2020)
K. Wu, C. Wang, X. Lang, J. Cheng, H. Wu, C. Lyu, W.-M. Lau, Z. Liang, X. Zhu, J. Zheng, Insight into selenium vacancies enhanced CoSe2/MoSe2 heterojunction nanosheets for hydrazine-assisted electrocatalytic water splitting. J. Colloid Interface Sci. 654, 1040–1053 (2024)
L. Mu, S. Qiu, G. Zhao, W. Liao, N. Zhao, X. Xu, A high-efficiency NiFeSe4/NiSe2 bifunctional electrocatalyst with outstanding oxygen evolution reaction and overall water splitting performance. J. Mater. Chem. A. 12, 1714 (2024)
S. Zhou, X. Chen, T. Li, Y. Wei, R. Sun, S. Han, J. Jiang, Heterogeneous interface engineering of cationic vacancy defects layered double hydroxides and molybdenum-nickel-based selenium compounds to facilitate overall water splitting. Fuel 357, 129732 (2024)
M. Bahrami, T. Shahrabi, Y. Yaghoubinezhad, Synergistic coupling of reduced graphene oxide with Ni0.85Se for highly active bifunctional electrocatalyst for water splitting. Int. J. Hydrogen Energy 53, 1421–1432 (2024)
A. Monshi, M.R. Foroughi, M.R. Monshi, Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano. Sci. Eng. 2, 154–160 (2012)
I.G. Shitu, Z.A. Talib, J.L.Y. Chi, M.M.A. Kechick, H. Baqiah, Influence of tartaric acid concentration on structural and optical properties of CuSe nanoparticles synthesized via microwave assisted method. Result Phys. 17, 103041 (2020)
Y.E. Firat, A. Peksoz, Efficiently two-stage synthesis and characterization of CuSe/Polypyrrole composite thin films. J. Alloy. Compd. 727, 177–184 (2017)
Acknowledgements
This work has been supported by the Researchers Supporting Project (RSP2024R405), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All have done equal contribution.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, S.I.A., Manzoor, S., Khan, M.M. et al. Development of CuSe/polypyrrole electrocatalyst for oxygen evolution reaction. Appl. Phys. A 130, 257 (2024). https://doi.org/10.1007/s00339-024-07429-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07429-3