Abstract
Objectives
Long-term toxicities of germ cell cancer (GCC) treatment are of particular importance in young men with a life expectancy of several decades after curative treatment. This study aimed to investigate the long-term effects of platinum-based chemotherapy on cardiac function and myocardial tissue in GCC survivors by cardiac magnetic resonance (CMR) imaging.
Methods
Asymptomatic GCC survivors ≥ 3 years after platinum-based chemotherapy and age-matched healthy controls underwent CMR assessment, including left ventricular (LV) and right ventricular (RV) ejection fraction (EF), strain analysis, late gadolinium enhancement (LGE) imaging, and T1/T2 map**.
Results
Forty-four survivors (age 44 [interquartile range, IQR 37–52] years; follow-up time 10 [IQR 5–15] years after chemotherapy) and 21 controls were evaluated. LV- and RVEF were lower in GCC survivors compared to controls (LVEF 56 ± 5% vs. 59 ± 5%, p = 0.017; RVEF 50 ± 7% vs. 55 ± 7%, p = 0.008). Seven percent (3/44) of survivors showed reduced LVEF (< 50%), and 41% (18/44) showed borderline LVEF (50–54%). The strain analysis revealed significantly reduced deformation compared to controls (LV global longitudinal strain [GLS] -13 ± 2% vs. -15 ± 1%, p < 0.001; RV GLS -15 ± 4% vs. -19 ± 4%, p = 0.005). Tissue characterization revealed focal myocardial fibrosis in 9 survivors (20%) and lower myocardial native T1 times in survivors compared to controls (1202 ± 25 ms vs. 1226 ± 37 ms, p = 0.016). Attenuated LVEF was observed after two cycles of platinum-based chemotherapy (54 ± 5% vs. 62 ± 5%, p < 0.001).
Conclusion
Based on CMR evaluation, combination chemotherapy with cumulative cisplatin ≥ 200 mg/m2 is associated with attenuated biventricular systolic function and myocardial tissue alterations in asymptomatic long-term GCC survivors.
Clinical relevance statement
Platinum-based chemotherapy is associated with decreased systolic function, non-ischemic focal myocardial scar, and decreased T1 times in asymptomatic long-term germ cell cancer survivors. Clinicians should be particularly aware of the risk of cardiac toxicity after platinum-based chemotherapy.
Key Points
• Platinum-based chemotherapy is associated with attenuation of biventricular systolic function, lower myocardial T1 relaxation times, and non-ischemic late gadolinium enhancement.
• Decreased systolic function and non-ischemic late gadolinium enhancement are associated with a cumulative cisplatin dose of ≥ 200 mg/m2.
• Cardiac MRI can help to identify chemotherapy-associated changes in cardiac function and tissue in asymptomatic long-term germ cell cancer survivors.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Germ-cell cancer (GCC) is the most common cancer in young men aged between 20 and 40 years. Due to an excellent sensitivity to platinum-based chemotherapy and the use of multimodal treatment approaches using chemotherapy and secondary tumor resection, cure rates of more than 90% can be achieved even in metastatic disease [1,2,3]. As a result, long-term toxicities after GCC treatment are of particular importance in this group of cancer survivors with a life expectancy of several decades.
Long-term sequelae of chemo- and radiotherapy for GCC include neuro-, pulmonary, and renal toxicity, an increased risk of secondary malignancy, hypogonadism, and infertility. Furthermore, GCC survivors are at increased risk for metabolic syndrome and cardiovascular disease [4]. Risk estimates for cardiovascular disease range from 1.3- to 5.7-fold after platinum-based chemotherapy compared to surgery only [5,6,7,8]. Therefore, optimization of cardiovascular risk factors is recommended in GCC survivorship care. However, knowledge about cardiac function and myocardial alterations in GCC survivors is limited, and no specific recommendations for cardiological examinations in GCC survivors do exist. Previous studies investigating cardiac function of GCC survivors using echocardiography described impaired diastolic function but no impairment of systolic function, except for one study that described decreased left ventricular ejection fraction (LVEF) in one out of 37 survivors after platinum-based treatment [9,10,11,12,13].
Echocardiography is widely used to monitor cardiac function in patients undergoing potentially cardiotoxic cancer treatment. Using strain analysis, early changes in cardiac contractility can be detected before LVEF changes are manifest [14, 15]. Strain imaging assesses the myocardial deformation capacity by quantifying the shortening of a myocardial segment in percent from end-diastole to end-systole. The assessment of LVEF and global longitudinal strain (GLS) using echocardiography is recommended in patients receiving a potentially cardiotoxic treatment [16, 17]. LV global circumferential strain (GCS) is also predictive of chemotherapy-related cardiotoxicity but has not been established as a screening parameter [18]. In case of insufficient imaging quality, cardiac magnetic resonance (CMR) imaging, which represents the gold standard for quantification of cardiac function and volumes, is recommended as an alternative imaging modality in evaluating cardiotoxicity [16, 19, 20]. The characterization of myocardial tissue by CMR may provide further information on cardiac changes during oncological treatment [19]. T2 map** identifies tissue inflammation characterized by edema, and T1 map** is used to detect interstitial fibrosis. Late gadolinium enhancement (LGE) can identify myocardial fibrosis and further differentiate ischemic from non-ischemic scar tissue based on the LGE patterns [20].
Therefore, this study aimed to gain insights into the long-term cardiac effects of platinum-based chemotherapy by characterizing cardiac function and myocardial tissue in GCC survivors using CMR imaging.
Methods
Study population
The study was approved by the local ethics committee (PV7173) and carried out in accordance with the Declaration of Helsinki. All participants gave written informed consent. Forty-six asymptomatic consecutive men with a history of GCC ≥ 3 years after completion of platinum-based chemotherapy were prospectively recruited between March 2020 and January 2022 and underwent a CMR scan. Survivors who had received radiotherapy were excluded. The general exclusion criteria were MRI contraindications, any history of coronary artery disease, heart failure, and arrhythmia. A historical cohort of 21 age-matched healthy men was used as a control cohort. One participant from the survivor group was excluded from the analysis because of extensive ischemic myocardial fibrosis due to silent myocardial infarction, and one patient withdrew consent due to claustrophobia (Fig. 1).
Anamnestic cardiovascular risk factors and medication were documented, and a laboratory analysis was performed, including NT-proBNP, Troponin I, cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), glomerular filtration rate (GFR), creatinine, and testosterone levels.
CMR protocol
CMR was performed on a 3.0-T scanner (Ingenia, Philips Medical Systems) as previously described [21]. Imaging was performed using a standard retrospective ECG-triggered steady-state free-precession (SSFP) cine sequence in short- and long-axis views with 25 cardiac phases. Native and post-contrast T1 map** was performed with a motion-corrected modified look-locker inversion (MOLLI) recovery sequence using the 5 s (3 s) 3 s scheme. T2 map** was performed with a free-breathing navigator-gated black-blood prepared gradient and spin-echo (GraSE) hybrid sequence in the basal, mid, and apical slices corresponding to the MOLLI sequence. LGE imaging with a phase-sensitive inversion recovery (PSIR) sequence was acquired 10 min after injecting 0.15 mmol/kg of gadoterate meglumine (Dotarem™).
CMR data analysis
CMR images were post-processed independently and blindly in random order by two investigators (E.T. and H.C.) using CVi42 software (Circle Cardiovascular Imaging Inc). The corresponding short-axis maps were used to carefully delineate endo- and epicardial contours with 10% endo- and epicardial offsets to avoid contamination. Evaluation of LV and RV volumes and LV mass was performed in standard fashion using short-axis cine images [22]. The presence of LGE was visually analyzed. LGE areas were excluded for T1 and ECV quantification. Extracellular volume (ECV) fraction was calculated using a previously validated equation [23]. CMR parameters are presented as the mean of two observers and indexed to the body surface area (BSA).
Myocardial strain values were generated based on cine CMR images using feature-tracking software Segment (version 2.1.R.6108, Medviso) [24]. GLS and global radial strain (GRS) were derived from three long-axis (2-, 3-, and 4-chamber views) cine series, whereas GCS was measured on three short-axis (apical, mid, and basal slices) cine series. Endo- and epicardial contours were manually delineated on end-diastolic images and were then automatically propagated by the software throughout the cardiac cycle, generating myocardial strain [24].
Statistical analysis
Statistical analysis was performed using SPSS for Windows, version 28.0.1.1 (IBM SPSS Statistics). Graphs were generated using ggplot2 via RStudio (version 2022.12.0.353). Demographic data are presented as median (range), continuous parameters as mean ± SD, and categorical data as absolute numbers and percentages. Normality of continuous data was assessed using the Shapiro–Wilk test. The Man-Whitney-U-Test was used for statistical analysis of demographic data, t-test for metric data, and Fisher’s exact test for categorical variables. Pearson’s correlation or Spearman’s correlation were applied as appropriate. Multivariate regression analyses were executed to analyze the effects of multiple variables on cardiac parameters with LVEF, LV GCS, and RV GCS, respectively, as the dependent variable. All p values are two-sided and considered significant at p < 0.05.
Results
Baseline demographics and tumor characteristics
Median age of survivors was 44 (interquartile range [IQR] 37–52) years. There were no significant differences in age, weight, height, body mass index (BMI), and BSA between survivors and controls (Table 1). The median follow-up time after completion of platinum-based chemotherapy was 10 (IQR 5–15) years (Table 2). Thirty-six percent of survivors had seminoma, and 64% had non-seminoma. The initial disease stage was I in 25%, II in 45%, and III in 30% of survivors, respectively (Table 2). In total, 33 (75%) survivors had received one, and 11 (25%) had received two or more lines of therapy as detailed in Table 2. A total of 10 survivors had received high-dose chemotherapy followed by autologous stem cell transplantation.
Cardiac function, volumes, and mass
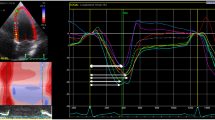
Detailed results of functional and volumetric CMR-derived parameters in survivors compared to controls are presented in Supplementary Table 1. LVEF and RVEF were significantly lower in GCC survivors compared to controls (LVEF 56 ± 5% vs. 59 ± 5%, p = 0.017; RVEF 50 ± 7% vs. 55 ± 7%, p = 0.008; Fig. 2a and b). Three survivors (7%) showed reduced LVEF below 50%, and 18 (41%) survivors presented with borderline LVEF between 50 and 54%. RV end-systolic volume index (RVESVi), a marker of ventricle remodeling, was significantly higher (45 ± 10 mL/m2 vs. 38 ± 11 mL/m2, p = 0.01) and there was a trend towards higher LVESVi in survivors compared to controls (39 ± 9 mL/m2 vs. 35 ± 10 mL/m2, p = 0.084; Supplementary Table 1). No significant differences were found for heart rate, LV cardiac index (cardiac output per minute related to BSA), LV mass index, atrial volumes, and ventricular systolic and ventricular end-diastolic volumes, respectively (Supplementary Table 1).
Systolic function in germ cell cancer survivors compared to age-matched controls. Cardiac magnetic resonance imaging was performed in asymptomatic germ cell cancer survivors after platinum-based chemotherapy (n = 44) and age-matched healthy controls (n = 21). For assessment of systolic function biventricular ejection fraction and myocardial strain was assessed: (a) left ventricular ejection fraction (LVEF), (b) right ventricular ejection fraction (RVEF), (c) LV global longitudinal strain (GLS), (d) RV GLS, (e) LV global circular strain (GCS), (f) RV GCS, (g) LV global radial strain (LV GRS), and (h) RV free wall longitudinal strain (FWLS). Data are presented as mean ± SD. T-test was performed
Myocardial strain
Strain analysis revealed significantly lower myocardial deformation of the left and right ventricle in survivors compared to controls as measured by GLS (LV GLS -13 ± 2% vs. -15 ± 1%, p < 0.001; RV GLS -15 ± 4% vs. -19 ± 4%, p = 0.005; Fig. 2c and d) and LV GCS (-14 ± 2% vs. -16 ± 2%, p < 0.001; Fig. 2e). No significant differences were found for RV GCS, LV radial strain, and RV free wall longitudinal strain (Fig. 2f–h).
Myocardial tissue characterization
Eight survivors (18%) showed non-ischemic LGE, indicating focal myocardial fibrosis (scar), and one survivor (2%) showed ischemic LGE (Fig. 3 and Supplementary Table 1). Four survivors with non-ischemic LGE had normal LVEF (≥ 55%), and 4 survivors with non-ischemic LGE had borderline LVEF (50–54%) (Table 3).
Non-ischemic LGE in germ cell cancer survivors. Myocardial tissue was characterized by cardiac magnetic resonance imaging in asymptomatic germ cell cancer survivors after platinum-based chemotherapy (n = 44) and age-matched healthy controls (n = 21) by late gadolinium enhancement (LGE) imaging. Short-axis and long-axis images of eight germ cell cancer survivors with non-ischemic LGE are shown. Arrows indicate non-ischemic LGE lesions. LGE lesions are predominantly located at subepicardial and mid-wall left ventricular segments. Two patients had LGE lesions located at the posterior right ventricular insertion point (survivors 3 and 8)
Myocardial native T1 map** revealed lower T1 relaxation times in survivors compared to controls (1202 ± 25 ms vs. 1226 ± 37 ms, p = 0.016; Fig. 4a). Survivors with decreased or borderline LVEF below 55% had lower myocardial T1 values when compared to those with LVEF ≥ 55% (1191 ± 22 ms vs. 1211 ± 25 ms, p = 0.007; Table 3). A moderate positive correlation was found between native T1 values and LVEF (r(41) = 0.346, p = 0.023), and RVEF (r(41) = 0.314, p = 0.04), respectively. There was a moderate negative correlation between native T1 values and LV GLS (r(41) = −0.341, p = 0.025), and a strong negative correlation between native T1 values and RV GLS (r(41) = −0.534, p < 0.001, Fig. 5), respectively. T2 values and ECV did not differ between groups (Fig. 4b and c). However, survivors with LVEF below 55% had significantly lower percentage of ECV compared with survivors with normal LVEF (24.2 ± 1.4% vs. 25.6 ± 1.9%, p = 0.008; Table 3).
Myocardial tissue alterations in germ cell cancer survivors. Myocardial tissue was characterized by cardiac magnetic resonance imaging in asymptomatic germ cell cancer survivors after platinum-based chemotherapy (n = 44) and age-matched healthy controls (n = 18) by T1/T2 map**. a Native T1 time, (b) native T2 time, and (c) extra cellular volume (ECV) were assessed. Data are presented as mean ± SD. T-Test was performed
Correlation of native myocardial T1 relaxation time and RV GLS. Myocardial tissue was characterized by cardiac magnetic resonance imaging in asymptomatic germ cell cancer survivors after platinum-based chemotherapy (n = 44) by T1/T2 map**. Pearson’s correlation was used to analyze the association of native myocardial T1 relaxation time and right ventricular global longitudinal strain (RV GLS)
Cardiac parameters in association with chemotherapy dose
Correlation of cumulative platinum dose and LVEF showed a drop in LVEF after merely two cycles of platinum-based combination chemotherapy (Fig. 6). Therefore, cardiac parameters and clinical characteristics were compared between survivors who received one cycle of chemotherapy (carboplatin AUC7 or cisplatin 100 mg/m2, n = 7) and those who received at least two cycles of platinum-based combination chemotherapy (cisplatin ≥ 200 mg/m2 ± carboplatin, n = 37).
LVEF impairment after two cycles of platinum-based chemotherapy. Graph shows left ventricular ejection fraction (LVEF) in germ cell cancer survivors (n = 44) measured by cardiac magnetic resonance imaging for subgroups depending on the number of platinum-based chemotherapy cycles received. T-test was applied
Survivors who had received ≥ 2 cycles of chemotherapy showed significantly higher body weight and BSA (Table 4). No differences were found for anamnestic cardiovascular risk factors or medication. The laboratory analysis revealed significantly impaired renal functional parameters and lower testosterone levels, and a trend towards higher cholesterol levels in survivors after ≥ 2 cycles of platinum-based chemotherapy. No significant differences were found for cardiac biomarkers Troponin I and NT-proBNP or ferritin values (Table 4). Two survivors who had received > 2 cycles of platinum-based combination chemotherapy showed elevated high-sensitive Troponin I. Both individuals presented with non-ischemic LGE not fulfilling criteria for acute myocarditis; acute myocardial infarction was ruled out in both individuals.
LVEF was significantly lower in survivors after ≥ 2 cycles of platinum-based combination chemotherapy compared to survivors who had received one cycle of chemotherapy (54 ± 5% vs. 62 ± 5%, p < 0.001; Table 4). Strain analysis showed a significant decrease in LV and RV GCS after ≥ 2 cycles of chemotherapy (LV GCS -13 ± 2% vs. -16 ± 2%, p < 0.001; RV GCS -8 ± 3% vs. -10 ± 2%, p = 0.046; Table 4), indicating decreased myocardial deformation after higher doses of chemotherapy. No significant changes were found for RVEF, LV GLS, and RV GLS, native T1 and T2 times, respectively. Notably, all survivors with focal LGE had received ≥ 2 cycles of platinum-based combination chemotherapy (Table 4). The multivariate regression analysis showed that the association of chemotherapy cycles with LVEF, LV GCS, and RV GCS, respectively, was independent of testosterone levels, renal function, body weight, and BSA.
Cardiac parameters did not differ between survivors who had received high-dose chemotherapy and autologous stem cell transplantation and those without high-dose chemotherapy.
Cardiac parameters in association with clinical and laboratory parameters
GCC survivors with anamnestic arterial hypertension had lower native T1 values (1179 ± 19 ms vs. 1207 ± 24 ms, p = 0.003). Anamnestic dyslipidemia or smoking status, age, and time of follow-up were not associated with differences in cardiac parameters. A weak negative correlation was found for LV GLS and HDL levels (r(42) = −0.347, p = 0.021). Beyond that, systolic functional parameters and T1 values did not correlate with levels of cholesterol, LDL, HDL, and testosterone, respectively.
Discussion
To the best of our knowledge, this is the first study to extensively characterize cardiac alterations in long-term survivors of GCC using CMR imaging. The study has three major findings: (1) We found attenuated systolic function in GCC survivors almost 10 years after therapy completion as measured by EF and strain analysis after only two cycles of platinum-based combination chemotherapy; (2) tissue characterization revealed that focal myocardial fibrosis detected by LGE was common in GCC survivors; and (3) survivors presented with mildly decreased native T1 values compared to controls.
Previous studies investigating the cardiac function of GCC survivors mainly used echocardiography. A recent study by Bjerring et al performed echocardiography including speckle-tracking derived strain analysis 30 years after cisplatin-based chemotherapy for GCC and found no differences in biventricular systolic function between survivors and controls [9]. The current study using CMR, including strain analysis based on a feature-tracking technique, demonstrated subclinical attenuation of systolic function after cisplatin-based chemotherapy after a median follow-up time of 10 years but not after one course of carboplatin. This functional impairment was observed after a threshold of two cycles of cisplatin-based combination chemotherapy (at ≥ 200 mg/m2 cumulative dose) without further dose-dependent decline beyond that, although data on this association are clearly still limited. These findings indicate that platinum-based chemotherapy has more extensive effects on the heart than known so far. This observation is supported by a previous in vivo study that described cisplatin-induced development of LV dysfunction and depressed cardiomyocyte contraction in mice that was associated with mitochondrial abnormalities, endoplasmic reticulum stress response, and apoptosis [25]. However, considering the results of Bjerring et al with a longer follow-up of 30 years, the observed subclinical changes in systolic function might not necessarily progress to clinical manifestation of heart failure [9]. In a population-based cohort study, mortality due to non-ischemic cardiac disease was increased in GCC survivors following combined chemo- and radiotherapy but not after chemotherapy alone [26]. Nevertheless, in the current study cohort 4 out of 45 asymptomatic survivors had decreased LVEF. Identifying patients with clinically asymptomatic structural and functional cardiac abnormalities provides an opportunity to initiate lifestyle modifications and pharmacological therapy that may prevent or delay progression to symptomatic heart failure [27]. Therefore, this study raises the question whether specific cardiological examinations should be included into the follow-up routine of GCC survivors after ≥ 200 mg/m2 cumulative cisplatin.
The current study revealed a high prevalence (18%) of focal myocardial fibrosis as identified by mid-wall/subepicardial LGE. Previous studies reported non-ischemic LGE in 4–9% of cancer patients and in 2.8–4% of healthy individuals [28,33]. Interestingly, we found that worse systolic functional parameters in survivors correlated with a decrease in native T1 values, pointing toward functional consequences of these tissue alterations. It remains unclear which myocardial tissue alterations underlie the detected native T1 decrease in GCC survivors. Ferritin values were within the normal range in this study cohort, excluding iron overload. Also, decreased T1 values, no differences in ECV compared to healthy controls, and lower ECV in survivors with LVEF below 55% argue against diffuse interstitial fibrosis in the myocardium of GCC survivors. Whether the reported decrease in T1 values is due to ultrastructural mitochondria abnormalities and accumulation of membranous debris within and dilation of the endoplasmatic reticulum in cardiomyocytes as described in cisplatin-treated mice remains to be elucidated.
Lower T1 values in patients with anamnestic arterial hypertension and association of lower HDL with worse LV GLS indicate that the observed functional changes and tissue alterations might be a secondary sequela. However, these associations were not robustly detected for all functional parameters. Based on this study, we cannot draw a conclusion on whether the observed cardiac effects are a result of direct chemotherapy-induced cardiotoxicity or rather a secondary result of metabolic changes or arterial hypertension.
Our study has some shortcomings. CMR is the gold standard for the assessment of systolic function and tissue characterization, a major limitation is that diastolic function cannot be validly assessed. Furthermore, this study does not allow to differentiate which chemotherapy agent is the cause of the observed cardiac effects. Even though almost every chemotherapy cycle contained a platinum agent, most survivors had also received several cycles of bleomycin and etoposide. However, survivors treated with only one cycle of carboplatin single agent did not seem to be at increased risk. The longitudinal studies with baseline CMR examinations and a longer follow-up are needed in order to elucidate the clinical significance and prognostic value of the subclinical systolic impairment observed in this cohort. Moreover, further studies are warranted to validate the finding of decreased T1 time in this survivorship group, as observed changes were rather small.
In conclusion, this study revealed for the first time that subclinical attenuation of LV and RV systolic function characterized by decreased biventricular ejection fraction and myocardial deformation, and changes in myocardial tissue characteristics can be detected in asymptomatic long-term GCC survivors following platinum-based chemotherapy. This study emphasizes the need for more in-depth investigations into the risk of cardiotoxicity associated with PEB (cisplatin/etoposide/bleomycin) chemotherapy and points to the importance of long-term cardiovascular follow-up in GCC survivors as well as education programs to avoid additional cardiac risk behavior in this survivorship population.
Abbreviations
- AUC:
-
Area under the curve
- BSA:
-
Body surface area
- CMR:
-
Cardiac magnetic resonance
- ECV:
-
Extracellular volume
- EF:
-
Ejection fraction
- GCC:
-
Germ cell cancer
- GCS:
-
Global circumferential strain
- GLS:
-
Global longitudinal strain
- GRS:
-
Global radial strain
- HDL:
-
High-density lipoprotein
- IQR:
-
Interquartile range
- LDL:
-
Low-density lipoprotein
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- RV:
-
Right ventricular
References
Hanna N, Einhorn LH (2014) Testicular cancer: a reflection on 50 years of discovery. J Clin Oncol 32:3085–3092
Beyer J, Collette L, Sauvé N et al (2021) Survival and new prognosticators in metastatic seminoma: results from the IGCCCG-update consortium. J Clin Oncol 39:1553–1562
Gillessen S, Sauvé N, Collette L et al (2021) Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): results from the IGCCCG update consortium. J Clin Oncol 39:1563–1574
Chovanec M, Lauritsen J, Bandak M et al (2021) Late adverse effects and quality of life in survivors of testicular germ cell tumour. Nat Rev Urol 18:227–245
Belt-Dusebout AWvd, Nuver J, Wit Rd et al (2006) Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol 24:467–475
Haugnes HS, Wethal T, Aass N et al (2010) Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol 28:4649–4657
Huddart RA, Norman A, Shahidi M et al (2003) Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol 21:1513–1523
Lauritsen J, Hansen MK, Bandak M et al (2020) Cardiovascular risk factors and disease after male germ cell cancer. J Clin Oncol 38:584–592
Bjerring AW, Fosså SD, Haugnes HS et al (2021) The cardiac impact of cisplatin-based chemotherapy in survivors of testicular cancer: a 30-year follow-up. Eur Heart J Cardiovasc Imaging 22:443–450
Altena R, Hummel YM, Nuver J et al (2011) Longitudinal changes in cardiac function after cisplatin-based chemotherapy for testicular cancer. Ann Oncol 22:2286–2293
Nuver J, Smit AJ, Sleijfer DT et al (2005) Left ventricular and cardiac autonomic function in survivors of testicular cancer. Eur J Clin Invest 35:99–103
Altena R, de Haas EC, Nuver J et al (2009) Evaluation of sub-acute changes in cardiac function after cisplatin-based combination chemotherapy for testicular cancer. Br J Cancer 100:1861–1866
van Schinkel LD, Willemse PM, van der Meer RW et al (2013) Chemotherapy for testicular cancer induces acute alterations in diastolic heart function. Br J Cancer 109:891–896
Negishi K, Negishi T, Haluska BA, Hare JL, Plana JC, Marwick TH (2014) Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging 15:324–331
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH (2014) Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 63:2751–2768
Lyon AR, López-Fernández T, Couch LS et al (2022) 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. https://doi.org/10.1093/eurheartj/ehac244
Liu JE, Barac A, Thavendiranathan P, Scherrer-Crosbie M (2020) Strain imaging in cardio-oncology. JACC CardioOncol 2:677–689
Narayan HK, French B, Khan AM et al (2016) Noninvasive measures of ventricular-arterial coupling and circumferential strain predict cancer therapeutics-related cardiac dysfunction. JACC Cardiovasc Imaging 9:1131–1141
Jeong D, Gladish G, Chitiboi T, Fradley MG, Gage KL, Schiebler ML (2019) MRI in cardio-oncology: a review of cardiac complications in oncologic care. J Magn Reson Imaging 50:1349–1366
Ponikowski P, Voors AA, Anker SD et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37:2129–2200
Tahir E, Azar M, Shihada S et al (2022) Myocardial injury detected by T1 and T2 map** on CMR predicts subsequent cancer therapy-related cardiac dysfunction in patients with breast cancer treated by epirubicin-based chemotherapy or left-sided RT. Eur Radiol 32:1853–1865
Schulz-Menger J, Bluemke DA, Bremerich J et al (2013) Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 15:35
Kellman P, Wilson JR, Xue H, Ugander M, Arai AE (2012) Extracellular volume fraction map** in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson 14:63
Morais P, Marchi A, Bogaert JA et al (2017) Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm: assessment of variability in a real-life clinical setting. J Cardiovasc Magn Reson 19:24
Ma H, Jones KR, Guo R, Xu P, Shen Y, Ren J (2010) Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin Exp Pharmacol Physiol 37:460–465
Hellesnes R, Myklebust TÅ, Fosså SD et al (2021) Testicular cancer in the cisplatin era: causes of death and mortality rates in a population-based cohort. J Clin Oncol 39:3561–3573
Heidenreich PA, Bozkurt B, Aguilar D et al (2022) 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 145:e876–e894
Modi K, Joppa S, Chen KA et al (2021) Myocardial damage assessed by late gadolinium enhancement on cardiovascular magnetic resonance imaging in cancer patients treated with anthracyclines and/or trastuzumab. Eur Heart J Cardiovasc Imaging 22:427–434
Domenech-**menos B, Sanz-de la Garza M, Prat-González S et al (2020) Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes. J Cardiovasc Magn Reson 22:62
Turkbey EB, Nacif MS, Guo M et al (2015) Prevalence and correlates of myocardial scar in a US cohort. JAMA 314:1945–1954
LotaAmrit S, Tsao A, Owen R et al (2021) Prognostic significance of nonischemic myocardial fibrosis in patients with normal LV volumes and ejection-fraction. JACC Cardiovasc Imaging 14:2353–2365
Muehlberg F, Funk S, Zange L et al (2018) Native myocardial T1 time can predict development of subsequent anthracycline-induced cardiomyopathy. ESC Heart Fail 5:620–629
Radenkovic D, Weingärtner S, Ricketts L, Moon JC, Captur G (2017) T1 map** in cardiac MRI. Heart Fail Rev 22:415–430
Acknowledgements
The authors wish to thank the survivors and healthy individuals who participated in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study has received funding from the Barbara and Wilfried Mohr Stiftung Hamburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Antonia Beitzen-Heineke.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Dr. Linda Krause (University Medical Center Hamburg-Eppendorf, Center for Experimental Medicine, Institute of Medical Biometry and Epidemiology) kindly provided statistical advice for this study.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained. The study was approved by the Ethics Committee of the Medical Association (Ärztekammer) Hamburg (PV7173) and carried out in accordance with the Declaration of Helsinki.
Study subjects or cohorts overlap
Not applicable.
Methodology
• prospective
• case–control study
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beitzen-Heineke, A., Rolling, C.C., Seidel, C. et al. Long-term cardiotoxicity in germ cell cancer survivors after platinum-based chemotherapy: cardiac MR shows impaired systolic function and tissue alterations. Eur Radiol 34, 4102–4112 (2024). https://doi.org/10.1007/s00330-023-10420-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10420-w