Abstract
Objectives
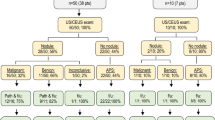
To identify the risk factors for predicting the malignant progression of LR-3/4 observations on the baseline and contrast-enhanced ultrasound (CEUS).
Methods
In total, 245 liver nodules assigned to LR-3/4 in 192 patients from January 2010 to December 2016 were followed up by baseline US and CEUS. The differences in the rate and time of progression to hepatocellular carcinoma (HCC) among subcategories (defined as P1-P7) of LR-3/4 in CEUS Liver Imaging Reporting and Data System (LI-RADS) were analyzed. The risk factors to predict progression to HCC were analyzed by univariate and multivariate Cox proportional hazard model analysis.
Results
A total of 40.3% of LR-3 nodules and 78.9% of LR-4 nodules eventually progressed to HCC. The cumulative incidence of progression was significantly higher for LR-4 than LR-3 (p < 0.001). The rate of progression was 81.2% in nodules with arterial phase hyperenhancement (APHE), 64.7% in nodules with late and mild washout, and 100% in nodules with both characteristics. The overall progression rate and median progression time of subcategory P1 nodules (LR-3a) were lower (38.0% vs. 47.6–100.0%) and later (25.1 months vs. 2.0–16.3 months) than those of other subcategories. The cumulative incidence of progression of LR-3a (P1), LR-3b (P2/3/4), and LR-4 (P5/6/7) categories were 38.0%, 52.9%, and 78.9%. The risk factors of HCC progression were Visualization score B/C, CEUS characteristics (APHE, washout), LR-4 classification, echo changes, and definite growth.
Conclusion
CEUS is a useful surveillance tool for nodules at risk of HCC. CEUS characteristics, LI-RADS classification, and changes in nodules provide useful information for the progress of LR-3/4 nodules.
Clinical relevance statement
CEUS characteristics, LI-RADS classification, and nodule changes provide important predictions for LR-3/4 nodule progression to HCC, which may stratify the risk of malignant progression to provide a more optimized and refined, more cost-effective, and time-efficient management strategy for patients.
Key Points
• CEUS is a useful surveillance tool for nodules at risk of HCC, CEUS LI-RADS successfully stratified the risks that progress to HCC.
• CEUS characteristics, LI-RADS classification, and changes in nodules can provide important information on the progression of LR-3/4 nodules, which may be helpful for a more optimized and refined management strategy.





Similar content being viewed by others
Abbreviations
- APHE :
-
Arterial phase hyperenhancement
- CEUS :
-
Contrast-enhanced ultrasound
- CI :
-
Confidence interval
- HCC :
-
Hepatocellular carcinoma
- HR :
-
Hazard ratio
- LI-RADS :
-
Liver Imaging Reporting and Data System
- TIV:
-
Tumor in vein
- US :
-
Ultrasound
- VIS :
-
Visualization Score
References
Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA (2014) Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 109:542–553
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Terzi E, Iavarone M, Pompili M et al (2018) Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter retrospective study of 1006 nodules. J Hepatol 68:485–492
Zheng W, Li Q, Zou XB et al (2020) Evaluation of contrast-enhanced US LI-RADS version 2017: application on 2020 liver nodules in patients with hepatitis B infection. Radiology 294:299–307
Santillan CS, Tang A, Cruite I, Shah A, Sirlin CB (2014) Understanding LI-RADS: a primer for practical use. Magn Reson Imaging Clin N Am 22:337–352
Mitchell DG, Bruix J, Sherman M, Sirlin CB (2015) LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 61:1056–1065
Kono Y, Lyshchik A, Cosgrove D et al (2017) Contrast enhanced ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS(R)): the official version by the American College of Radiology (ACR). Ultraschall Med 38:85–86
Wilson SR, Lyshchik A, Piscaglia F et al (2018) CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY) 43:127–142
American College of Radiology (2018) Liver imaging reporting and data system version 2017. American College of Radiology. Available via https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CEUS-LI-RADS. Accessed 26 Dec 2018
Beal EW, Albert S, McNally M et al (2014) An indeterminate nodule in the cirrhotic liver discovered by surveillance imaging is a prelude to malignancy. J Surg Oncol 110:967–969
Sato T, Kondo F, Ebara M et al (2015) Natural history of large regenerative nodules and dysplastic nodules in liver cirrhosis: 28-year follow-up study. Hepatol Int 9:330–336
Kondo F, Ebara M, Sugiura N et al (1990) Histological features and clinical course of large regenerative nodules: evaluation of their precancerous potentiality. Hepatology 12(3 Pt 1):592–598
Ng CH, Chan SW, Lee WK et al (2011) Hepatocarcinogenesis of regenerative and dysplastic nodules in Chinese patients. Hong Kong Med J 17(1):11–19
Efremidis SC, Hytiroglou P (2002) The multistep process of hepatocarcinogenesis in cirrhosis with imaging correlation. Eur Radiol 12(4):753–764
Burke LM, Sofue K, Alagiyawanna M et al (2016) Natural history of liver imaging reporting and data system category 4 nodules in MRI. Abdom Radiol (NY) 41:1758–1766
Sofue K, Burke LMB, Nilmini V et al (2017) Liver imaging reporting and data system category 4 observations in MRI: risk factors predicting upgrade to category 5. J Magn Reson Imaging 46:783–792
Tanabe M, Kanki A, Wolfson T et al (2016) Imaging outcomes of liver imaging reporting and data system version 2014 category 2, 3, and 4 observations detected at CT and MR imaging. Radiology 281(1):129–139
Hong CW, Park CC, Mamidipalli A et al (2019) Longitudinal evolution of CT and MRI LI-RADS v2014 category 1, 2, 3, and 4 observations. Eur Radiol 29:5073–5081
Atri M, Jang HJ, Kim TK, Khalili K (2022) Contrast-enhanced US of the Liver and Kidney: A Problem-solving Modality. Radiology 211347
Chiorean L, Tana C, Braden B et al (2016) Advantages and limitations of focal liver lesion assessment with ultrasound contrast agents: comments on the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Guidelines. Med Princ Pract 25:399–407
Pace C, Nardone V, Roma S et al (2019) Evaluation of contrast-enhanced intraoperative ultrasound in the detection and management of liver lesions in patients with hepatocellular carcinoma. J Oncol 2019:6089340
Romanini L, Passamonti M, Aiani L et al (2007) Economic assessment of contrast-enhanced ultrasonography for evaluation of focal liver lesions: a multicentre Italian experience. Eur Radiol 17(Suppl 6):F99–F106
Nolsoe CP, Lorentzen T (2016) International guidelines for contrast-enhanced ultrasonography: ultrasound imaging in the new millennium. Ultrasonography 35:89–103
American College of Radiology (2018) Ultrasound LI-RADS v2017. American College of Radiology. Available via https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/LI-RADS-Ultrasound-v2017. Accessed 11 Nov 2018
American College of Radiology (2018) Liver imaging reporting and data system version 2018. American College of Radiology. Available via https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS. Accessed 2 Sep 2018
Min YW, Gwak GY, Lee MW et al (2012) Clinical course of sub-centimeter-sized nodules detected during surveillance for hepatocellular carcinoma. World J Gastroenterol 18:2654–2660
Choi HH, Perez MG, Millet JD et al (2019) Association of advanced hepatic fibrosis and sonographic visualization score: a dual-center study using ACR US LI-RADS. Abdom Radiol (NY) 44(4):1415–1422
Funding
This study was supported by the National Nature Science Foundation of China (NO: 82171960, 82102078, and 81971630), Guangdong Natural Science Foundation (NO: 2021B1515020054, 2022A1515011148, 2021B1515120030), Guangzhou Science and Technology Project (NO: 201904010187), and Guangdong Medical Scientific Research Foundation (NO: A2021344).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Li-da Chen.
Conflict of interest
There are no conflicts of interest to declare.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
-
retrospectively
-
diagnostic or prognostic study
-
performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, H., Li, Cq., He, Dn. et al. Surveillance for malignant progression of LI-RADS version 2017 category 3/4 nodules using contrast-enhanced ultrasound. Eur Radiol 33, 9336–9346 (2023). https://doi.org/10.1007/s00330-023-09811-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09811-w