Abstract
Objective
To investigate the findings of magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), and serum metabolomics for differentiating pre-eclampsia (PE) from gestational hypertension (GH).
Methods
This prospective study enrolled 176 subjects including a primary cohort with healthy non-pregnant women (HN, n = 35), healthy pregnant women (HP, n = 20), GH (n = 27), and PE (n = 39) and a validation cohort with HP (n = 22), GH (n = 22), and PE (n = 11). T1 signal intensity index (T1SI), apparent diffusion coefficient (ADC) value, and the metabolites on MRS were compared. The differentiating performances of single and combined MRI and MRS parameters for PE were evaluated. Serum liquid chromatography-mass spectrometry (LC–MS) metabolomics was investigated by sparse projection to latent structures discriminant analysis.
Results
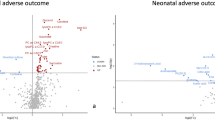
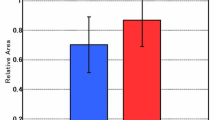
Increased T1SI, lactate/creatine (Lac/Cr), and glutamine and glutamate (Glx)/Cr and decreased ADC value and myo-inositol (mI)/Cr in basal ganglia were found in PE patients. T1SI, ADC, Lac/Cr, Glx/Cr, and mI/Cr yielded an area under the curves (AUC) of 0.90, 0.80, 0.94, 0.96, and 0.94 in the primary cohort, and of 0.87, 0.81, 0.91, 0.84, and 0.83 in the validation cohort, respectively. A combination of Lac/Cr, Glx/Cr, and mI/Cr yielded the highest AUC of 0.98 in the primary cohort and 0.97 in the validation cohort. Serum metabolomics analysis showed 12 differential metabolites, which are involved in pyruvate metabolism, alanine metabolism, glycolysis, gluconeogenesis, and glutamate metabolism.
Conclusions
MRS is expected to be a noninvasive and effective tool for monitoring GH patients to avoid the development of PE.
Key Points
• Increased T1SI and decreased ADC value in the basal ganglia were found in PE patients than in GH patients.
• Increased Lac/Cr and Glx/Cr, and decreased mI/Cr in the basal ganglia were found in PE patients than in GH patients.
• LC–MS metabolomics showed that the major differential metabolic pathways between PE and GH were pyruvate metabolism, alanine metabolism, glycolysis, gluconeogenesis, and glutamate metabolism.





Similar content being viewed by others
Abbreviations
- 1H-NMR:
-
1H nuclear magnetic resonance spectroscopy
- Cr:
-
Creatine
- GH:
-
Gestational hypertension
- Gln:
-
Glutamine
- HDP:
-
Hypertensive disorders of pregnancy
- Lac:
-
Lactate
- LC-MS:
-
Liquid chromatography-mass spectrometry
- mI:
-
Myo-inositol
- MRS:
-
Magnetic resonance spectroscopy
- PE:
-
Pre-eclampsia
- sPLS-DA:
-
Sparse projection to latent structures discriminant analysis
- T1SI:
-
T1 signal intensity index
References
Tsakiridis I, Giouleka S, Arvanitaki A et al (2021) Gestational hypertension and preeclampsia: An overview of national and international guidelines. Obstet Gynecol Surv 76:613–633
Haram K, Mortensen JH, Mastrolia SA, Erez O (2017) Disseminated intravascular coagulation in the HELLP syndrome: how much do we really know? J Matern Fetal Neonatal Med 30:779–788
Auer J, Camoin L, Guillonneau F et al (2010) Serum profile in preeclampsia and intra-uterine growth restriction revealed by iTRAQ technology. J Proteomics 73:1004–1017
Bozkurt M, Yumru AE, Sahin L, Salman S (2015) Troponin I and D-Dimer levels in preeclampsia and eclampsia: prospective study. Clin Exp Obstet Gynecol 42:26–31
Salam RA, Das JK, Ali A, Bhaumik S, Lassi ZS (2015) Diagnosis and management of preeclampsia in community settings in low and middle-income countries. J Family Med Prim Care 4:501–506
Pongrojpaw D, Chanthasenanont A, Nanthakomon T (2010) Second trimester uterine artery Doppler screening in prediction of adverse pregnancy outcome in high risk women. J Med Assoc Thai 7:127–130
Hinchey J, Chaves C, Appignani B et al (1996) A reversible posterior leukoencephalopathy syndrome. N Engl J Med 334:494–500
Di X, Mai H, Zheng Z, Guo K, Morse AN, Liu H (2018) Neuroimaging findings in women who develop neurologic symptoms in severe preeclampsia with or without eclampsia. Hypertens Res 41:598–604
Brewer J, Owens MY, Wallace K et al (2013) Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol 208:468.e1-e6
Nelander M, Hannsberger D, Sundström-Poromaa I et al (2018) Assessment of cerebral perfusion and edema in preeclampsia with intravoxel incoherent motion MRI. Acta Obstet Gynecol Scand 97:1212–1218
Nicholson JK, Lindon JC, Holmes E (1999) ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29:1181–1189
Nelander M, Wikström AK, Weis J et al (2018) Cerebral osmolytes and plasma osmolality in pregnancy and preeclampsia: a proton magnetic resonance spectroscopy study. Am J Hypertens 31:847–853
Rutherford JM, Moody A (2003) Crawshaw S, Magnetic resonance spectroscopy in pre-eclampsia: evidence of cerebral ischaemia. BJOG 110:416–423
Turner E, Brewster JA, Simpson NAB, Walker JJ, Fisher J (2007) Plasma from women with preeclampsia has a low lipid and ketone body content–a nuclear magnetic resonance study. Hypertens Pregnancy 26:329–342
Odibo AO, Goetzinger KR, Odibo L et al (2011) First-trimester prediction of preeclampsia using metabolomic biomarkers: a discovery phase study. Prenat Diagn 31:990–994
Rohart F, Gautier B, Singh A, LeCao KA (2017) mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol 13:e1005752
Casey SO, Sampaio RC, Michel E, Truwit CL (2000) Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol 21:1199–1206
Postma IR, Slager S, Kremer HP, De Groot JC, Zeeman GG (2014) Long-term consequences of the posterior reversible encephalopathy syndrome in eclampsia and preeclampsia: a review of the obstetric and nonobstetric literature. Obstet Gynecol Surv 69:287–300
Demirtas O, Gelal F, Vidinli BD, Demirtas LO, Uluc E, Baloğlu A (2005) Cranial MR imaging with clinical correlation in preeclampsia and eclampsia. Diagn Interv Radiol 11:189–194
Li SJ, Jiang L, Fu X et al (2014) Pallidal index as biomarker of manganese brain accumulation and associated with manganese levels in blood: a meta-analysis. PLoS One 9:e93900
Sarwar MS, Ahmed S, Ullah MS et al (2013) Comparative study of serum zinc, copper, manganese, and iron in preeclamptic pregnant women. Biol Trace Elem Res 154:14–20
Vigeh M, Yokoyama K, Ramezanzadeh F et al (2006) Lead and other trace metals in preeclampsia: a case-control study in Tehran. Iran Environ Res 100:268–275
Fitsanakis VA, Zhang N, Avison MJ, Erikson KM, Gore JC, Aschner M (2011) Changes in dietary iron exacerbate regional brain manganese accumulation as determined by magnetic resonance imaging. Toxicol Sci 120:146–153
Li Y, Mei L, Qiang J, Ju S, Zhao S (2016) Magnetic resonance spectroscopy for evaluating portal-systemic encephalopathy in patients with chronic hepatic schistosomiasis Japonicum. PLoS Negl Trop Dis 10:e0005232
Li Y, Qiang JW, Ju S (2013) Brain MR imaging changes in patients with hepatic schistosomiasis japonicum without liver dysfunction. Neurotoxicology 35:101–105
Nelander M, Weis J, Bergman L, Larsson A, Wikström AK, Wikström J (2017) Cerebral magnesium levels in preeclampsia; a phosphorus magnetic resonance spectroscopy study. Am J Hypertens 30:667–672
Euser AG, Cipolla MJ (2009) Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke 40:1169–1175
McKinney AM, Filice RW, Teksam M et al (2004) Diffusion abnormalities of the globi pallidi in manganese neurotoxicity. Neuroradiology 46:291–295
Distefano G, Praticò AD (2010) Actualities on molecular pathogenesis and repairing processes of cerebral damage in perinatal hypoxic-ischemic encephalopathy. Ital J Pediatr 36:63
Zwingmann C, Leibfritz D, Hazell AS (2003) Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J Cereb Blood Flow Metab 23:756–771
Flo K, Blix ES, Husebekk A et al (2016) A longitudinal study of maternal endothelial function, inflammatory response and uterine artery blood flow during the second half of pregnancy. Acta Obstet Gynecol Scand 95:225–232
Ewertsen C, Kondziella D, Danielsen ER, Thomsen C (2015) Good outcome after posterior reversible encephalopathy syndrome (PRES) despite elevated cerebral lactate: a case report. Acta Radiol Open 4:2058460115578324
Takado Y, Sato N, Kanbe Y et al (2019) Association between brain and plasma glutamine levels in healthy young subjects investigated by MRS and LC/MS. Nutrients. https://doi.org/10.3390/nu11071649
Andersen JV, Nissen JD, Christensen SK, Markussen KH, Waagepetersen HS (2017) Impaired hippocampal glutamate and glutamine metabolism in the db/db mouse model of type 2 diabetes mellitus. Neural Plast 2017:2107084
Albrecht J, Norenberg MD (2006) Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology 44:788–794
Rutherford JM, Moody A, Crawshaw S, Rubin PC (2003) Magnetic resonance spectroscopy in pre-eclampsia: evidence of cerebral ischaemia. BJOG 110:416–423
Miller BL, Chang L, Booth R et al (1996) In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci 58:1929–1935
Mcmahon KE, Farrell PM (1985) Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin Chim Acta 149:1–12
van der Grond J, Balm R, Kappelle LJ, Eikelboom BC, Mali WP (1995) Cerebral metabolism of patients with stenosis or occlusion of the internal carotid artery. A 1H-MR spectroscopic imaging study. Stroke 26:822–828
Magnotta VA, Friedman L, FIRST BIRN, (2006) Measurement of signal-to-noise and contrast-to-noise in the fBIRN multicenter imaging study. J Digit Imaging 19:140–147
Acknowledgements
We also thank Chun-Hua Guo from Shanghai Sensichip Infotech Co. Ltd, Shanghai, China, for the assistance with metabolomics data analysis.
Funding
This study has received funding from Shanghai Municipal Health Commission (No. ZK2019B01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantors of this publication are Ying LI and **-Wei QIANG.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, XF., Li, MD., Lu, JJ. et al. Magnetic resonance spectroscopy and liquid chromatography-mass spectrometry metabolomics study may differentiate pre-eclampsia from gestational hypertension. Eur Radiol 33, 4554–4563 (2023). https://doi.org/10.1007/s00330-023-09454-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09454-x