Abstract
Objectives
To investigate and characterize the structural alterations of the brain in SCA3, and their correlations with the scale for the assessment and rating of ataxia (SARA) and normal brain ATXN3 expression.
Methods
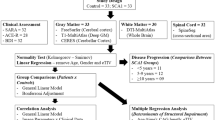
We performed multimodal analyses in 52 SCA3 (15 pre-symptomatic) and healthy controls (HCs) (n = 35) to assess the abnormalities of gray and white matter (WM) of the cerebrum, brainstem, and cerebellum via FreeSurfer, SUIT, and TBSS, and their associations with disease severity. Twenty SCA3 patients (5 pre- and 15 symptomatic) were followed for at least a year. Besides, we uncovered the normal pattern of brain ATXN3 spatial distribution.
Results
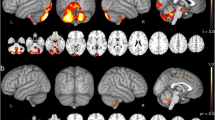
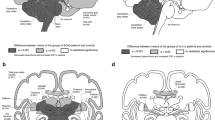
Pre-symptomatic patients showed only WM damage, mainly in the cerebellar peduncles, compared to HCs. In the advanced stage, the WM damage followed a caudal-rostral pattern. Meanwhile, continuous nonlinear structure damage was characterized by brainstem volumetric reduction and relatively symmetric cerebellar and basal ganglia atrophy but spared the cerebral cortex, partially explained by the ATXN3 overexpression. The bilateral pallidum, brainstem, and cerebellar peduncles demonstrated a very large effect size. Besides, all these alterations were significantly correlated with SARA; the pons (r = −0.65) and superior cerebellar peduncle (r = −0.68) volume demonstrated a higher correlation than the cerebellum with SARA. The longitudinal study further uncovered progressive atrophy of pons in symptomatic SCA3.
Conclusions
Significant WM damage starts before the ataxia onset. The bilateral pallidum, brainstem, and cerebellar peduncles are the most vulnerable targets. The volume of pons appears to be the most promising imaging biomarker for a longitudinal study.
Trial registration
ClinicalTrial ID: ChiCTR2100045857 (http://www.chictr.org.cn/edit.aspx?pid=55652&htm=4)
Key Points
• Pre- SCA3 showed WM damage mainly in cerebellar peduncles. Continuous brain damage was characterized by brainstem, widespread, and relatively symmetric cerebellar and basal ganglia atrophy.
• Volumetric abnormalities were most evident in the bilateral pallidum, brainstem, and cerebellar peduncles in SCA3.
• The volume of pons might identify the disease progression longitudinally.






Similar content being viewed by others
Abbreviations
- CAG:
-
Cytosine-adenine-guanine
- DKI:
-
Diffusion kurtosis imaging
- ES:
-
Effect size
- FDR:
-
False discovery rate
- GM:
-
Gray matter
- HCs:
-
Healthy controls
- ICP:
-
Inferior cerebellar peduncles
- Kr:
-
Radial kurtosis
- SARA:
-
Scale for the assessment and rating of ataxia
- SCA3:
-
Spinocerebellar ataxia type 3
- SCP:
-
Superior cerebellar peduncles
- TBSS:
-
Tract-based Spatial Statistics
- WM:
-
White matter
References:
Cancel GAN, Stevanin G, Dürr A et al (1995) Marked phenotypic heterogeneity associated with expansion of a CAG repeat sequence at the spinocerebellar atazxia 3 Machado-Joseph disease locus. Am J Hum Genet 4:809–816
Costa MDC (2020) Recent therapeutic prospects for Machado-Joseph disease. Curr Opin Neurol 33:519–526
Furtado GV, Oliveira CM, Bolzan G, Saute JAM, Saraiva-Pereira ML, Jardim LB (2019) State biomarkers for Machado Joseph disease: validation, feasibility and responsiveness to change. Genet Mol Biol 42:238–251
Adanyeguh IM, Perlbarg V, Henry P-G et al (2018) Autosomal dominant cerebellar ataxias: Imaging biomarkers with high effect sizes. Neuroimage Clin 19:858–867
Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rüb U (2012) Brain pathology of spinocerebellar ataxias. Acta Neuropathol 124(1):1–21
Koeppen AH (2018) The neuropathology of spinocerebellar ataxia type 3/Machado-Joseph disease. In: Nóbrega C, Pereira de Almeida L (eds) Polyglutamine disorders. Springer International Publishing, Cham, pp 233–241
Joers JM, Deelchand DK, Lyu T et al (2018) Neurochemical abnormalities in premanifest and early spinocerebellar ataxias. Ann Neurol 83:816–829
Faber J, Schaprian T, Berkan K et al (2021) Regional brain and spinal cord volume loss in spinocerebellar ataxia type 3. Mov Disord 36:2273–2281
Wan N, Chen Z, Wan L, Tang B, Jiang H (2020) MR Imaging of SCA3/MJD. Front Neurosci 14:749
de Rezende TJ, D'Abreu A, Guimaraes RP et al (2015) Cerebral cortex involvement in Machado-Joseph disease. Eur J Neurol 22(277-283):e223–e274
Park YW, Joers JM, Guo B et al (2020) Assessment of cerebral and cerebellar white matter microstructure in spinocerebellar ataxias 1, 2, 3, and 6 using diffusion MRI. Front Neurol 11:411
Arruda WO, Meira AT, Ono SE et al (2020) Volumetric MRI changes in spinocerebellar ataxia (SCA3 and SCA10) patients. Cerebellum 19:536–543
Jacobi H, Reetz K, du Montcel ST et al (2013) Biological and clinical characteristics of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 in the longitudinal RISCA study: analysis of baseline data. Lancet Neurol 12:650–658
Rezende TJR, de Paiva JLR, Martinez ARM et al (2018) Structural signature of SCA3: from presymptomatic to late disease stages. Ann Neurol 84:401–408
Wu X, Liao X, Zhan Y et al (2017) Microstructural alterations in asymptomatic and symptomatic patients with spinocerebellar ataxia type 3: a tract-based spatial statistics study. Front Neurol 8:714
Akcimen F, Ross JP, Liao C, Spiegelman D, Dion PA, Rouleau GA (2020) Expanded CAG Repeats in ATXN1, ATXN2, ATXN3, and HTT in the 1000 Genomes Project. Mov Disord. https://doi.org/10.1002/mds.28341
de Mattos EP, Leotti VB, Soong BW et al (2019) Age at onset prediction in spinocerebellar ataxia type 3 changes according to population of origin. Eur J Neurol 26:113–120
Gan SR, Figueroa KP, Xu HL et al (2020) The impact of ethnicity on the clinical presentations of spinocerebellar ataxia type 3. Parkinsonism Relat Disord 72:37–43
Billiet T, Vandenbulcke M, Madler B et al (2015) Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiol Aging 36:2107–2121
Cheung J, Doerr M, Hu R, Sun PZ (2020) Refined ischemic penumbra imaging with tissue ph and diffusion kurtosis magnetic resonance imaging. Transl Stroke Res. https://doi.org/10.1007/s12975-020-00868-z
Zhao J, Wang YL, Li XB et al (2019) Comparative analysis of the diffusion kurtosis imaging and diffusion tensor imaging in grading gliomas, predicting tumour cell proliferation and IDH-1 gene mutation status. J Neurooncol 141:195–203
Arribarat G, De Barros A, Peran P (2020) Modern brainstem MRI techniques for the diagnosis of Parkinson’s disease and Parkinsonisms. Front Neurol 11:791
Maas RPPWM, van Gaalen J, Klockgether T, van de Warrenburg BPC (2015) The preclinical stage of spinocerebellar ataxias. Neurology 85:96-103
Servelhere KR, Rezende TJR, de Lima FD et al (2021) Brain damage and gene expression across hereditary spastic paraplegia subtypes. Mov Disord 36:1644–1653
Desikan RS, Segonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980
Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N (2009) A probabilistic MR atlas of the human cerebellum. Neuroimage 46:39–46
Diedrichsen J, Zotow E (2015) Surface-based display of volume-averaged cerebellar imaging data. PLoS One 10:e0133402
Schmitz-Hübsch T, Fimmers R, Rakowicz M et al (2010) Responsiveness of different rating instruments in spinocerebellar ataxia patients. Neurology 74:678-684
Wang PS, Wu YT, Wang TY, Wu HM, Soong BW, Jao CW (2020) Supratentorial and infratentorial lesions in spinocerebellar ataxia type 3. Front Neurol 11:124
Li M, Chen X, Xu HL et al (2021) Brain structural abnormalities in the preclinical stage of Machado-Joseph disease/spinocerebellar ataxia type 3 (MJD/SCA3): evaluation by MRI morphometry, diffusion tensor imaging and neurite orientation dispersion and density imaging. J Neurol. https://doi.org/10.1007/s00415-021-10890-2
Kang JS, Klein JC, Baudrexel S, Deichmann R, Nolte D, Hilker R (2014) White matter damage is related to ataxia severity in SCA3. J Neurol 261:291–299
Kelm ND, West KL, Carson RP, Gochberg DF, Ess KC, Does MD (2016) Evaluation of diffusion kurtosis imaging in ex vivo hypomyelinated mouse brains. Neuroimage 124:612–626
Nakata Y, Sakamoto A, Kawata A (2020) Neuromelanin imaging analyses of the substantia nigra in patients with Machado-Joseph disease. Neuroradiology 62:1433–1439
Piccinin CC, Rezende TJR, de Paiva JLR et al (2020) A 5-year longitudinal clinical and magnetic resonance imaging study in spinocerebellar ataxia type 3. Mov Disord 35:1679–1684
Funding
This study has received funding from the National Natural Science Foundation of China (82172015) and the Guangdong Basic and Applied Basic Research Foundation, China (2022A1515011264, 2021A1515012279, and 2020A1515011436).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Chu Jian**.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haishan Qiu is the first author, and Chao Wu and Jiahui Liang are the co-first authors.
Supplementary information
ESM 1
(DOCX 53 kb)
About this article
Cite this article
Qiu, H., Wu, C., Liang, J. et al. Structural alterations of spinocerebellar ataxias type 3: from pre-symptomatic to symptomatic stage. Eur Radiol 33, 2881–2894 (2023). https://doi.org/10.1007/s00330-022-09214-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09214-3