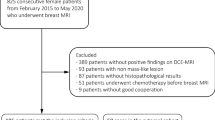
Abstract
Objectives
To evaluate the diagnostic performance of Kaiser score (KS) adjusted with the apparent diffusion coefficient (ADC) (KS+) and machine learning (ML) modeling.
Methods
A dataset of 402 malignant and 257 benign lesions was identified. Two radiologists assigned the KS. If a lesion with KS > 4 had ADC > 1.4 × 10−3 mm2/s, the KS was reduced by 4 to become KS+. In order to consider the full spectrum of ADC as a continuous variable, the KS and ADC values were used to train diagnostic models using 5 ML algorithms. The performance was evaluated using the ROC analysis, compared by the DeLong test. The sensitivity, specificity, and accuracy achieved using the threshold of KS > 4, KS+ > 4, and ADC ≤ 1.4 × 10−3 mm2/s were obtained and compared by the McNemar test.
Results
The ROC curves of KS, KS+, and all ML models had comparable AUC in the range of 0.883–0.921, significantly higher than that of ADC (0.837, p < 0.0001). The KS had sensitivity = 97.3% and specificity = 59.1%; and the KS+ had sensitivity = 95.5% with significantly improved specificity to 68.5% (p < 0.0001). However, when setting at the same sensitivity of 97.3%, KS+ could not improve specificity. In ML analysis, the logistic regression model had the best performance. At sensitivity = 97.3% and specificity = 65.3%, i.e., compared to KS, 16 false-positives may be avoided without affecting true cancer diagnosis (p = 0.0015).
Conclusion
Using dichotomized ADC to modify KS to KS+ can improve specificity, but at the price of lowered sensitivity. Machine learning algorithms may be applied to consider the ADC as a continuous variable to build more accurate diagnostic models.
Key Points
• When using ADC to modify the Kaiser score to KS+, the diagnostic specificity according to the results of two independent readers was improved by 9.4–9.7%, at the price of slightly degraded sensitivity by 1.5–1.8%, and overall had improved accuracy by 2.6–2.9%.
• When the KS and the continuous ADC values were combined to train models by machine learning algorithms, the diagnostic specificity achieved by the logistic regression model could be significantly improved from 59.1 to 65.3% (p = 0.0015), while maintaining at the high sensitivity of KS = 97.3%, and thus, the results demonstrated the potential of ML modeling to further evaluate the contribution of ADC.
• When setting the sensitivity at the same levels, the modified KS+ and the original KS have comparable specificity; therefore, KS+ with consideration of ADC may not offer much practical help, and the original KS without ADC remains as an excellent robust diagnostic method.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under ROC curve
- BI-RADS:
-
Breast Imaging Reporting and Data System
- BPE:
-
Background parenchymal enhancement
- CI:
-
Confidence interval
- DCE:
-
Dynamic contrast-enhanced
- DCIS:
-
Ductal carcinoma in situ
- DWI:
-
Diffusion-weighted imaging
- ICC:
-
Intraclass correlation coefficient
- KS:
-
Kaiser score
- LDA:
-
Linear discriminant analysis
- LR:
-
Logistic regression
- ML:
-
Machine learning
- MRI:
-
Magnetic resonance imaging
- NB:
-
Naive Bayes
- ROC:
-
Receiver operator characteristic
- ROI:
-
Region of interest
- SVM_L:
-
Linear kernel support vector machine
- SVM_R:
-
Radial kernel support vector machine
- VIBRANT:
-
Volume imaging for breast assessment
References
Mann RM, Cho N, Moy L (2019) Breast MRI: state of the art. Radiology 292:520–536
Mann RM, Kuhl CK, Moy L (2019) Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging 50:377–390
Sumkin JH, Berg WA, Carter GJ et al (2019) Diagnostic performance of MRI, molecular breast imaging, and contrast-enhanced mammography in women with newly diagnosed breast cancer. Radiology 293:531–540
D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA (2013) ACR BI-RADS atlas, breast imaging reporting and data system. American College of Radiology, Reston
Eghtedari M, Chong A, Rakow-Penner R, Ojeda-Fournier H (2021) Current status and future of BI-RADS in multimodality imaging, from the AJR Special Series on Radiology Reporting and Data Systems. AJR Am J Roentgenol 216:860–873
Rawashdeh M, Lewis S, Zaitoun M, Brennan P (2018) Breast lesion shape and margin evaluation: BI-RADS based metrics understate radiologists’ actual levels of agreement. Comput Biol Med 96:294–298
Pinker K, Moy L, Sutton EJ et al (2018) Diffusion-weighted imaging with apparent diffusion coefficient map** for breast cancer detection as a stand-alone parameter: comparison with dynamic contrast-enhanced and multiparametric magnetic resonance imaging. Invest Radiol 53:587–595
Dietzel M, Baltzer PAT (2018) How to use the Kaiser score as a clinical decision rule for diagnosis in multiparametric breast MRI: a pictorial essay. Insights Imaging 9:325–335
Baltzer PA, Dietzel M, Kaiser WA (2013) A simple and robust classification tree for differentiation between benign and malignant lesions in MR-mammography. Eur Radiol 23:2051–2060
Baltzer P, Toth D Breast MRI lesion classification tree (Kaiser score). Available at: http://www.meduniwien.ac.at/kaiser-score/. Accessed 6 Sept 2020
Milos RI, Pipan F, Kalovidouri A et al (2020) The Kaiser score reliably excludes malignancy in benign contrast-enhancing lesions classified as BI-RADS 4 on breast MRI high-risk screening exams. Eur Radiol 30:6052–6061
Marino MA, Clauser P, Woitek R et al (2016) A simple scoring system for breast MRI interpretation: does it compensate for reader experience? Eur Radiol 26:2529–2537
Wengert GJ, Pipan F, Almohanna J et al (2020) Impact of the Kaiser score on clinical decision-making in BI-RADS 4 mammographic calcifications examined with breast MRI. Eur Radiol 30:1451–1459
Jajodia A, Sindhwani G, Pasricha S et al (2021) Application of the Kaiser score to increase diagnostic accuracy in equivocal lesions on diagnostic mammograms referred for MR mammography. Eur J Radiol 134:109413
Le Bihan D (2003) Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4:469–480
Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R (2006) Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics 26(Suppl 1):S205–S223
Bickel H, Pinker-Domenig K, Bogner W et al (2015) Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Invest Radiol 50:95–100
Woodhams R, Matsunaga K, Iwabuchi K et al (2005) Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr 29:644–649
Baltzer A, Dietzel M, Kaiser CG, Baltzer PA (2016) Combined reading of contrast enhanced and diffusion weighted magnetic resonance imaging by using a simple sum score. Eur Radiol 26:884–891
Dietzel M, Krug B, Clauser P et al (2021) A multicentric comparison of apparent diffusion coefficient map** and the Kaiser score in the assessment of breast lesions. Invest Radiol 56:274–282
Meng L, Zhao X, Lu L et al (2021) A comparative assessment of MR BI-RADS 4 breast lesions with Kaiser score and apparent diffusion coefficient value. Front Oncol 11:779642
Baltzer P, Mann RM, Iima M et al (2020) Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur Radiol 30:1436–1450
Cicchetti DV (1994) Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 6:284–290
Istomin A, Masarwah A, Vanninen R, Okuma H, Sudah M (2021) Diagnostic performance of the Kaiser score for characterizing lesions on breast MRI with comparison to a multiparametric classification system. Eur J Radiol 138:109659
Baltzer PAT, Kaiser WA, Dietzel M (2015) Lesion type and reader experience affect the diagnostic accuracy of breast MRI: a multiple reader ROC study. Eur J Radiol 84:86–91
Clauser P, Krug B, Bickel H et al (2021) Diffusion-weighted imaging allows for downgrading MR BI-RADS 4 lesions in contrast-enhanced MRI of the breast to avoid unnecessary biopsy. Clin Cancer Res 27:1941–1948
Kul S, Metin Y, Kul M, Metin N, Eyuboglu I, Ozdemir O (2018) Assessment of breast mass morphology with diffusion-weighted MRI: beyond apparent diffusion coefficient. J Magn Reson Imaging 48:1668–1677
Kawashima H, Miyati T, Ohno N et al (2018) Differentiation between phyllodes tumours and fibroadenomas using intravoxel incoherent motion magnetic resonance imaging: comparison with conventional diffusion-weighted imaging. Br J Radiol 91:20170687
Pinker K, Bickel H, Helbich TH et al (2013) Combined contrast-enhanced magnetic resonance and diffusion-weighted imaging reading adapted to the “Breast Imaging Reporting and Data System” for multiparametric 3-T imaging of breast lesions. Eur Radiol 23:1791–1802
Daimiel Naranjo I, Lo Gullo R, Saccarelli C et al (2021) Diagnostic value of diffusion-weighted imaging with synthetic b-values in breast tumors: comparison with dynamic contrast-enhanced and multiparametric MRI. Eur Radiol 31:356–367
Ei Khouli RH, Jacobs MA, Mezban SD et al (2010) Diffusion-weighted imaging improves the diagnostic accuracy of conventional 3.0-T breast MR imaging. Radiology 256:64–73
Baltzer PAT, Bickel H, Spick C et al (2018) Potential of noncontrast magnetic resonance imaging with diffusion-weighted imaging in characterization of breast lesions: intraindividual comparison with dynamic contrast-enhanced magnetic resonance imaging. Invest Radiol 53:229–235
Zhang M, Horvat JV, Bernard-Davila B et al (2019) Multiparametric MRI model with dynamic contrast-enhanced and diffusion-weighted imaging enables breast cancer diagnosis with high accuracy. J Magn Reson Imaging 49:864–874
Kim SY, Lee HS, Kim EK, Kim MJ, Moon HJ, Yoon JH (2016) Effect of background parenchymal enhancement on pre-operative breast magnetic resonance imaging: how it affects interpretation and the role of second-look ultrasound in patient management. Ultrasound Med Biol 42:2766–2774
DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD (2012) Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR Am J Roentgenol 198:W373–W380
Horvat JV, Durando M, Milans S et al (2018) Apparent diffusion coefficient map** using diffusion-weighted MRI: impact of background parenchymal enhancement, amount of fibroglandular tissue and menopausal status on breast cancer diagnosis. Eur Radiol 28:2516–2524
Funding
This work was supported by the Key Laboratory of Intelligent Medical Imaging of Wenzhou (No. 2021HZSY0057), the Key Laboratory of Alzheimer’s Disease of Zhejiang Province, Institute of Aging, Wenzhou Medical University, Wenzhou, Zhejiang, China, Wenzhou Science & Technology Bureau (No. Y20180185), the Medical Health Science and Technology Project of Zhejiang Province Health Commission (No. 2019KY102), the National Cancer Institute of the National Institutes of Health under award numbers P30 CA062203, R01 CA127927, and R21 CA208938, and the UC Irvine Comprehensive Cancer Center using UCI Anti-Cancer Challenge funds.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Min-Ying Su, PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board of the First Affiliated Hospital of Wenzhou Medical University.
Ethical approval
Institutional Review Board approval was obtained from Institutional Review Board of the First Affiliated Hospital of Wenzhou Medical University (approval number 2021-R007).
Methodology
• retrospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, ZW., Zhao, YF., Liu, HR. et al. Assessment of breast lesions by the Kaiser score for differential diagnosis on MRI: the added value of ADC and machine learning modeling. Eur Radiol 32, 6608–6618 (2022). https://doi.org/10.1007/s00330-022-08899-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08899-w