Abstract
Objective
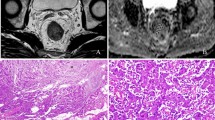
To investigate the diagnostic performance of the apparent diffusion coefficient (ADC) derived from intratumoral and peritumoral zones for assessing pathologic prognostic factors in rectal cancer.
Materials and methods
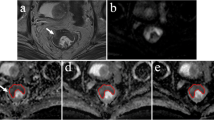
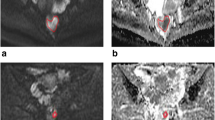
One hundred forty-six patients with rectal cancer who underwent preoperative MRI were prospectively enrolled. Two radiologists independently placed free-hand regions of interest (ROIs) in the largest tumor cross section and three small ROIs on the peritumoral zone adjacent to the tumor contour. Maximum values of tumor ADC (ADCtmax), minimum values of tumor ADC (ADCtmin), mean values of tumor ADC (ADCtmean), mean values of peritumor ADC (ADCpmean), and ADCpmean/ADCtmean (ADC ratio) were obtained on ADC maps and correlated with prognostic factors using uni- and multivariate logistic regression, and receiver operating characteristic curve (ROC) analysis.
Results
Interobserver agreement was excellent for ADCtmax and ADCtmean (intraclass correlation coefficient [ICC], 0.915–0.958), and were good for ADCtmin, ADCpmean, and ADC ratio (ICC, 0.774–0.878). The ADC ratio was significantly higher in the poor differentiation, T3–4 stage, lymph node metastasis (LNM)–positive, extranodal extension (ENE)–positive, tumor deposit (TD)–positive, and lymphovascular invasion (LVI)–positive groups than that in the well–moderate differentiation, T1–2 stage, LNM-negative, ENE-negative, TD-negative, and LVI-negative groups (p = 0.008, < 0.001, < 0.001, 0.001, < 0.001, and < 0.001, respectively). The area under the ROC curve (AUC) of the ADC ratio was the highest for assessing poor differentiation (0.700), T3–4 stage (0.707), LNM-positive (0.776), TD-positive (0.848), and LVI-positive (0.778). Both the ADC ratio (AUC = 0.677) and ADCpmean (AUC = 0.686) showed higher diagnostic performance for assessing ENE.
Conclusion
The ADC ratio could provide better predictive performance for assessing preoperative prognostic factors in resectable rectal cancer.
Key Points
• Both the peritumor/tumor ADC ratio and ADC pmean are correlated with important prognostic factors of resectable rectal cancer.
• Both peritumor ADC and peritumor/tumor ADC ratio had higher diagnostic performance than tumor ADC for assessment of prognostic factors in resectable rectal cancer.
• Peritumor/tumor ADC ratio showed the most capability for the assessment of prognostic factors in resectable rectal cancer.





Similar content being viewed by others
Abbreviations
- ADC ratio:
-
ADCpmean/ADCtmean
- ADCpmean :
-
Mean values of peritumor ADC
- ADCtmax :
-
Maximum values of tumor ADC
- ADCtmean :
-
Mean values of tumor ADC
- ADCtmin :
-
Minimum values of tumor ADC
- AUCs:
-
Areas under the receiver operating characteristic curves
- CI:
-
Confidence interval
- ENE:
-
Extranodal extension
- ICC:
-
Intraclass correlation coefficient
- LNM:
-
Lymph node metastasis
- LVI:
-
Lymphovascular invasion
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SD:
-
Standard deviations
- TD:
-
Tumor deposit
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL, Tumour and Angiogenesis Research Group (2005) Inclusion of vasculature-related variables in the Dukes staging system of colon cancer. Clin Cancer Res 11:8653–8660
Bown EJ, Lloyd GM, Boyle KM, Miller AS (2014) Rectal cancer: prognostic indicators of long-term outcome in patients considered for surgery. Int J Colorectal 29:147–155
Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK (2017) AJCC cancer staging manual, 8th edn. New York, NY: Springer.
Glynne-Jones R, Wyrwicz L, Tiret E et al (2017) Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv22–iv40
Diagnosis And Treatment Guidelines For Colorectal Cancer Working Group CSOCOC (2019) Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for colorectal cancer 2018 (English version). Chin J Cancer Res 31:117–134
Mirkin KA, Kulaylat AS, Hollenbeak CS, Messaris E (2018) Prognostic significance of tumor deposits in stage III colon cancer. Ann Surg Oncol 25:3179–3184
Wind J, Lagarde SM, Ten Kate FJ, Ubbink DT, Bemelman WA, van Lanschot JJ (2007) A systematic review on the significance of extracapsular lymph node involvement in gastrointestinal malignancies. Eur J Surg Oncol 33:401–408
Yamano T, Semba S, Noda M et al (2015) Prognostic significance of classified extramural tumor deposits and extracapsular lymph node invasion in T3-4 colorectal cancer: a retrospective single-center study. BMC Cancer 15:859
Kim CW, Kim J, Park Y et al (2019) Prognostic implications of extranodal extension in relation to colorectal cancer location. Cancer Res Treat 51:1135–1143
Horvat N, Carlos TRC, Clemente OB, Petkovska I, Gollub MJ (2019) MRI of rectal cancer: tumor staging, imaging techniques, and management. Radiographics 39:367–387
Brown G, Richards CJ, Bourne MW et al (2003) Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 227:371–377
Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG (2004) High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 52:78–83
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622–1635
Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E (2004) Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev 18:2095–2107
Mori N, Mugikura S, Takasawa C et al (2016) Peritumoral apparent diffusion coefficients for prediction of lymphovascular invasion in clinically node-negative invasive breast cancer. Eur Radiol 26:331–339
Hu Y, **e C, Yang H et al (2020) Assessment of intratumoral and peritumoral computed tomography radiomics for predicting pathological complete response to neoadjuvant chemoradiation in patients with esophageal squamous cell carcinoma. JAMA Netw Open 3:e2015927
Deng L, Wang QP, Yan R et al (2018) The utility of measuring the apparent diffusion coefficient for peritumoral zone in assessing infiltration depth of endometrial cancer. Cancer Imaging 18:23
Liu J, Li Q, Tang L, Huang Z, Lin Q (2021) Correlations of mean and minimum apparent diffusion coefficient values with the clinicopathological features in rectal cancer. Acad Radiol 28:S105–S111
Li H, Chen GW, Liu YS et al (2020) Assessment of histologic prognostic factors of resectable rectal cancer: comparison of diagnostic performance using various apparent diffusion coefficient parameters. Sci Rep 10:11554
Kim YI, Cho H, Kim CW et al (2021) Prognostic impact of extranodal extension in rectal cancer patients undergoing radical resection after preoperative chemoradiotherapy. Clin Colorectal Cancer 20:e35–e42
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Giavarina D (2015) Understanding Bland Altman analysis. Biochem Med (Zagreb) 25:141–151
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG (2012) Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging 35:1365–1371
Sun Y, Tong T, Cai S, Bi R, **n C, Gu Y (2014) Apparent diffusion coefficient (ADC) value: a potential imaging biomarker that reflects the biological features of rectal cancer. PLoS One 9:e109371
Orel VE, Ashykhmin A, Golovko T, Rykhalskyi O, Orel VB (2021) Texture analysis of tumor and peritumoral tissues based on 18F-fluorodeoxyglucose positron emission tomography/computed tomography hybrid imaging in patients with rectal cancer. J Comput Assist Tomogr 45:820–828
Chen LD, Li W, **an MF et al (2020) Preoperative prediction of tumour deposits in rectal cancer by an artificial neural network-based US radiomics model. Eur Radiol 30:1969–1979
Uematsu T, Kasami M, Watanabe J (2014) Is evaluation of the presence of prepectoral edema on T2-weighted with fat suppression 3 T breast MRI a simple and readily available noninvasive technique for estimation of prognosis in patients with breast cancer? Breast Cancer 21:684–692
Cheon H, Kim HJ, Kim TH et al (2018) Invasive breast cancer: prognostic value of peritumoral edema identified at preoperative MR imaging. Radiology 287:68–75
Braithwaite AC, Dale BM, Boll DT, Merkle EM (2009) Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology 250:459–465
Kettunen T, Okuma H, Auvinen P et al (2020) Peritumoral ADC values in breast cancer: region of interest selection, associations with hyaluronan intensity, and prognostic significance. Eur Radiol 30:38–46
Acknowledgements
The authors acknowledge Siyun Liu from General Electric Healthcare and Zhenlin Li from The West China Hospital for their great support for the statistical analysis. This study was approved by the Sichuan Provincial People’s Hospital institutional review board. Approval from our institutional animal care committee was not applicable because this is a human research.
Funding
This study has received funding from Sichuan Science and Technology Program (grant number, 2020YFH0166) and the Key Research Project of Sichuan Province (grant number, 2022YFS0249).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Hang Li.
Conflict of interest
The authors of this manuscript declare that Siyun Liu is a statistician from GE Healthcare and controls of the study data. The other authors declare no competing interests.
Statistics and biometry
Siyun Liu and Zhenlin Li kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, Y., Chen, Xl., Li, Zl. et al. The application of apparent diffusion coefficients derived from intratumoral and peritumoral zones for assessing pathologic prognostic factors in rectal cancer. Eur Radiol 32, 5106–5118 (2022). https://doi.org/10.1007/s00330-022-08717-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08717-3