Abstract
The aim of this study is to explain the consequences of the occurrence of enthalpy–entropy compensation (EEC) and isoequilibrium relationship during slow pyrolysis of cigarette butt filters (CBFs), consisting cellulose acetate (AC) as the main component. By using model-free and model-based kinetic methods and thermodynamic calculations, the complete reaction mechanism and extrathermodynamic issues about investigated process are completely resolved. It was established that compensation phenomenon where isoequilibrium temperature occurs is a consequence of formation of low-entropy molecular structure (cellulose II) and breaking of hydrogen bonds. Consequently, it was concluded that mechanisms which include a formation of low-entropy molecular structure and H-bonds breakage enter the changes in both enthalpy and entropy, which compensate each other. For the cellulose II generation, it was necessary to invest energy which represents use up energy. This “wasted” energy turns into work for creation of cellulose II molecular structures, whereby additional energy was provided from “local” decomposition reactions (which were identified in the established process mechanism) that arise from pyrolysis of starting material. Therefore, it was concluded that for “local reactions”, a negative ΔS° value was identified, but for the global process, the entropy change retained a positive value. Considering thermodynamic effects manifested through compensation temperature found below glass transition temperature (Tg) in the undercooling conditions, it was concluded that small amounts of plasticizers affect reduction of free volume in the glassy state. Obtained results indicated a decrease in β-relaxation mode of cellulose acetate by an abatement of the polymer free volume.
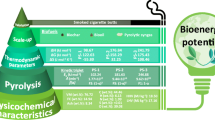
Graphical abstract

















Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Hamzai L, Novotny TE, Hendrix L, Williams R (2022) The Cigarette Filter: A Review of Utility, Environmental Impacts, and Policy Solutions (Appendix B1), pp. 1–45. Available at: https://merg.sdsu.edu.
Marinello S, Lolli F, Gamberini R, Rimini B (2020) A second life for cigarette butts? A review of recycling solutions. J Haz Mater 384:121245. https://doi.org/10.1016/j.jhazmat.2019.121245
Novotny TE, Lum K, Smith E, Wang V, Barnes R (2009) Cigarettes butts and the case for an environmental policy on hazardous cigarette waste. Int J Environ Res Pub Health 6(5):1691–1705. https://doi.org/10.3390/ijerph6051691
Wypych G (2017) Plasticizers Use and Selection for Specific Polymers. In: Wypych G (ed) Handbook of Plasticizers, 3rd edn. ChemTec Publishing Elsevier Inc
Sayers EW, Barrett T, Benson DA et al (2011) Database resources of the National Center for Biotechnology Information. Nuc Acids Res 39(1):D38–D51. https://doi.org/10.1093/nar/gkq1172
Gopi S, Pius A, Kargl R, Kleinschek KS, Sabu T (2019) Fabrication of cellulose acetate/chitosan blend films as efficient adsorbent for anionic water pollutants. Polym Bull 76:1557–1571. https://doi.org/10.1007/s00289-018-2467-y
Sevilla M, Díez N, Fuertes AB (2021) Cellulose as a precursor of high-performance energy storage materials in Li-S batteries and supercapacitors. Energy Technol Gener Conver Storage Distrib 9(8):2100268. https://doi.org/10.1002/ente.202100268
Reis DT, Ribeiro IHS, Pereira DH (2020) DFT study of the application of polymers cellulose and cellulose acetate for adsorption of metal ions (Cd2+, Cu2+ and Cr3+) potentially toxic. Polym Bull 77:3443–3456. https://doi.org/10.1007/s00289-019-02926-5
Long Y, Yu Y, Chua YW, Wu H (2017) Acid-catalysed cellulose pyrolysis at low temperatures. Fuel 193:460–466. https://doi.org/10.1016/j.fuel.2016.12.067
Lu Q, Wu Y, Hu B et al (2019) Insight into the mechanism of secondary reactions in cellulose pyrolysis: interactions between levoglucosan and acetic acid. Cellulose 26:8279–8290. https://doi.org/10.1007/s10570-019-02466-1
Shraah AA, Helleur R (2014) Microwave pyrolysis of cellulose at low temperature. J Anal Appl Pyrol 105:91–99. https://doi.org/10.1016/j.jaap.2013.10.007
Zhang X, Li J, Yang W, Blasiak W (2011) Formation mechanism of levoglucosan and formaldehyde during cellulose pyrolysis. Energy Fuels 25(8):3739–3746. https://doi.org/10.1021/ef2005139
Hazbehiean M, Mokhtarian N, Hallajisani A (2022) Converting the cigarette butts into valuable products using the pyrolysis process. Global J Environ Sci Manag 8(1):133–150. https://doi.org/10.22034/gjesm.2022.01.10
Wang W, Wang M, Huang J et al (2020) High efficiency pyrolysis of used cigarette filters for ester-rich bio-oil through microwave-assisted heating. J Clean Prod 257:120596. https://doi.org/10.1016/j.jclepro.2020.120596
Soltani SM, Yazdi SK, Hosseini S (2014) Effects of pyrolysis conditions on the porous structure construction of mesoporous charred carbon from used cigarette filters. Appl Nanosci 4:551–569. https://doi.org/10.1007/s13204-013-0230-0
Yousef S, Eimontas J, Striūgas N et al (2022) Pyrolysis kinetic behaviour, TG-FTIR, and GC/MS analysis of cigarette butts and their components. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02698-5
Alves JLF, da Silva JCG, Mumbach GD et al (2022) Thermo-kinetic investigation of the multi-step pyrolysis of smoked cigarette butts towards its energy recovery potential. Biomass Conv Bioref 12:741–755. https://doi.org/10.1007/s13399-020-01077-2
De Fenzo A, Giordano M, Sansone L (2020) A clean process for obtaining high-quality cellulose acetate from cigarette butts. Materials 13(21):4710. https://doi.org/10.3390/ma13214710
Lee B (1994) Enthalpy-entropy compensation in the thermodynamics of hydrophobicity. Biophys Chem 51(2–3):271–278. https://doi.org/10.1016/0301-4622(94)00048-4
Kumar D, Poša M (2023) Linear hydrophobic congeneric groups of bile acid anion derivatives based on the self-association (micellization) process and the phenomenon of enthalpy-entropy compensation. J Mol Liq 382:121925. https://doi.org/10.1016/j.molliq.2023.121925
Puls J, Wilson SA, Hölter D (2011) Degradation of cellulose acetate-based materials: A review. J Polym Environ 19:152–165. https://doi.org/10.1007/s10924-010-0258-0
PDF-2, Release 2023, International Crystallographical Database (ICDD), N.S. 12 Campus Blvd, PA 19073, USA, Editor. 2012: USA.
Vyazovkin S, Wight CA (1999) Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta 340–341:53–68. https://doi.org/10.1016/S0040-6031(99)00253-1
Koga N, Vyazovkin S, Burnham AK et al (2023) ICTAC Kinetics Committee recommendations for analysis of thermal decomposition kinetics. Thermochim Acta 719:179384. https://doi.org/10.1016/j.tca.2022.179384
Sharma A, Sharma V (2023) Forensic analysis of cigarette filter using non-destructive ATR-FTIR spectroscopy and chemometric methods. Forensic Chem 32:100465. https://doi.org/10.1016/j.forc.2023.100465
Glugoski LP, de Jesus CP, Fujiwara ST (2017) Reactive black 5 dye degradation using filters of smuggled cigarette modified with Fe3+. Environ Sci Poll Res 24:6143–6150. https://doi.org/10.1007/s11356-016-6820-0
Guo Y, Wu P (2008) Investigation of the hydrogen-bond structure of cellulose diacetate by two-dimensional infrared correlation spectroscopy. Carbohyd Polym 74:509–513. https://doi.org/10.1016/j.carbpol.2008.04.005
Marchessault RH, Liang CY (1960) Infrared spectra of crystalline polysaccharides. III Mercerized cellulose J Polym Sci 43(141):71–84. https://doi.org/10.1002/pol.1960.1204314107
Cichosz S, Masek A, Wolski K, Zaborski M (2019) Universal approach of cellulose fibres chemical modification result analysis via commonly used techniques. Polym Bull 76:2147–2162. https://doi.org/10.1007/s00289-018-2487-7
Homem NC, Amorim MTP (2020) Synthesis of cellulose acetate using as raw material textile wastes. Mater Today Proceed 31(2):S315–S317. https://doi.org/10.1016/j.matpr.2020.01.494
Lyu Y, Asoh T-A, Uyama H (2021) Fabrication of inorganic oxide fiber using a cigarette filter as a template. ACS Omega 6(23):15374–15381. https://doi.org/10.1021/acsomega.1c01750
Manurung R, Saputra A, Inarto H, Siregar AGA (2022) Esterification of refined glycerol biodiesel by-product using heterogeneous catalyst polystyrene sulfonic acid waste-based EPS foam. Rasayan J Chem 15(3):2053–2058. https://doi.org/10.31788/RJC.2022.1536933
Arroyo FD, Castro-Guerrero CF, León-Silva U (2020) Thin films of cellulose acetate nanofibers from cigarette butt waste. Prog Rubber Plast Recyc Technol 36(1):3–17. https://doi.org/10.1177/1477760619895024
Asriza RO, Ropalia HD, Ryaldi GO, Zomi, (2021) Characterization of cellulose acetate functional groups synthesized from corn husk (Zea mays). IOP Conf Ser Earth Environ Sci 926:012060. https://doi.org/10.1088/1755-1315/926/1/012060
Manríquez-Ramírez ME, Elizalde I, Ramírez-López R, Trejo-Valdez M, Estrada-Flores M (2020) Acetylation of glycerol using MgO-CaO catalysts with different CaO loadings. React Kinet Mechan Cat 130:417–431. https://doi.org/10.1007/s11144-020-01774-z
Cichosz S, Masek A (2019) Cellulose fibers hydrophobization via a hybrid chemical modification. Polymers 11:1174. https://doi.org/10.3390/polym11071174
Bao C (2015) Cellulose acetate/plasticizer systems: Structure, morphology and dynamics. Polymers. Université Claude Bernard - Lyon I, English, NNT:2015LYO10049, pp. 4–180.
Daud WRW, Djuned FM (2015) Cellulose acetate from oil palm empty fruit bunch via a one step heterogeneous acetylation. Carbohyd Polym 132:252–260. https://doi.org/10.1016/j.carbpol.2015.06.011
Phuong VT, Lazzeri A (2012) ‘“Green”’ biocomposites based on cellulose diacetate and regenerated cellulose microfibers: Effect of plasticizer content on morphology and mechanical properties. Compos A Appl Sci Manuf 43(12):2256–2268. https://doi.org/10.1016/j.compositesa.2012.08.008
Barud HS, de Araújo Júnior AM, Santos DB et al (2008) Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose. Thermochim Acta 471:61–69. https://doi.org/10.1016/j.tca.2008.02.009
Hatakeyama T, Hatakeyama H (2004) Thermal Properties of Cellulose and its Derivatives – Ch. 3, In: Thermal Properties of Green Polymers and Biocomposites. Part of the Book Series: Hot Topics in Thermal Analysis and Calorimetry (HTTC, Vol. 4), Springer, Kluwer Academic Publishers, pp. 39–130.
Watanabe A, Morita S, Ozaki Y (2006) Study on temperature-dependent changes in hydrogen bonds in cellulose Iβ by infrared spectroscopy with perturbation-correlation moving-window two-dimensional correlation spectroscopy. Biomacromol 7(11):3164–3170. https://doi.org/10.1021/bm0603591
Mankar SS, Chaudhari AR, Kalambe AB (2016) Miscibility and thermal studies of cellulose acetate and modified industrial waste lignin blends. Int J Innov Appl Res 4(1):45–55
Leguy J (2018) Periodate oxidation of cellulose for internal plasticization and materials design. Material chemistry. Université Grenoble Alpes, English, NNT: 2018GREAV010, pp. 22–23.
Gordon M, Taylor JS (1952) Ideal copolymers and the second-order transitions of synthetic rubbers. i. Non-crystalline copolymers. J Appl Chem 2(9):493–500. https://doi.org/10.1002/jctb.5010020901
Phuong VT, Verstichel S, Cinelli P et al (2014) Cellulose acetate blends - Effect of plasticizers on properties and biodegradability. J Renew Mater 2(1):35–41. https://doi.org/10.7569/JRM.2013.634136
Decroix C, Chalamet Y, Sudre G, Caroll V (2020) Thermo-mechanical properties and blend behaviour of cellulose acetate/lactates and acid systems: Natural-based plasticizers. Carbohyd Polym 237:116072. https://doi.org/10.1016/j.carbpol.2020.116072
Tatavarti AS, Dollimore D, Alexander KS (2002) A thermogravimetric analysis of non-polymeric pharmaceutical plasticizers: Kinetic analysis, method validation, and thermal stability evaluation. AAPS PharmSci 4(4):231–242. https://doi.org/10.1208/ps040445
Laino T, Tuma C, Moor P et al (2012) Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A 116(18):4602–4609. https://doi.org/10.1021/jp300997d
Morgado DL, Frollini E (2011) Thermal decomposition of mercerized linter cellulose and its acetates obtained from a homogeneous reaction. Polímeros 21(2):111–117. https://doi.org/10.1590/S0104-14282011005000025
Heinze T, Liebert T (2012) 10.05 - Celluloses and polyoses/hemicelluloses. Polym Sci A Comp Ref 10:83–152. https://doi.org/10.1016/B978-0-444-53349-4.00255-7
Hiller LA Jr (1953) The reaction of cellulose acetate with acetic acid and water. J Polym Sci 10(4):385–423. https://doi.org/10.1002/pol.1953.120100404
Sousa M, Brás AR, Veiga HIM et al (2010) Dynamical characterization of a cellulose acetate polysaccharide. J Phys Chem B 114(34):10939–10953. https://doi.org/10.1021/jp101665h
Yadav N, Hakkarainen M (2021) Degradable or not? Cellulose acetate as a model for complicated interplay between structure, environment and degradation. Chemosphere 265:128731. https://doi.org/10.1016/j.chemosphere.2020.128731
Greco G, Videgain M, Di Stasi C et al (2021) Importance of pyrolysis temperature and pressure in the concentration of polycyclic aromatic hydrocarbons in wood waste-derived biochars. J Anal Appl Pyrolysis 159:105337. https://doi.org/10.1016/j.jaap.2021.105337
L’vov BV, Galwey AK (2013) Interpretation of the kinetic compensation effect in heterogeneous reactions: thermochemical approach. Int Rev Phys Chem 32(4):515–557. https://doi.org/10.1080/0144235X.2013.802109
Mianowski A, Radko T, Siudyga T (2021) Kinetic compensation effect of isoconversional methods. React Kin Mech Catal 132:37–58. https://doi.org/10.1007/s11144-020-01898-2
Mianowski A, Urbańczyk W (2018) Isoconversional methods in thermodynamic principles. J Phys Chem A 122(34):6819–6828. https://doi.org/10.1021/acs.jpca.8b04432
Ioelovich M (2014) Cellulose: Nanostructured Natural Polymer. LAP LAMBERT Academic Publishing
van Krevelen DW, te Nijenhuis K (1972) Properties of Polymers. Their Correlation with Chemical Structure: Their Numerical Estimation and Prediction from Additive Group Contributions. 1st Ed., Elsevier B.V., Amsterdam, London, New York, pp. 129–189.
Ioelovich M (2016) Isophase transitions of cellulose - A short review. Athens J Sci 3(4):309–322. https://doi.org/10.30958/ajs.3-4-4
Jenckel E, Heusch R (1953) Die Erniedrigung der Einfriertemperatur organischer Gläser durch Lösungsmittel. Kolloïd Z Z Polym 130:89–105
Kalogeras IM, Brostow W (2009) Glass transition temperatures in binary polymer blends. J Polym Sci B Polym Phys 47(1):80–95. https://doi.org/10.1002/polb.21616
Stanford VL, McCulley CM, Vyazovkin S (2016) Isoconversional kinetics of nonisothermal crystallization of salts from solutions. J Phys Chem B 120(25):5703–5709. https://doi.org/10.1021/acs.jpcb.6b03860
Seddiqi H, Oliaei E, Honarkar H et al (2021) Cellulose and its derivatives: towards biomedical applications. Cellulose 28:1893–1931. https://doi.org/10.1007/s10570-020-03674-w
Zuber M, Zia KM, Bhatti IA et al (2012) Modification of cellulosic fibers by UV-irradiation. Part II: After treatments effects. Int J Biol Macromol 51:743–748. https://doi.org/10.1016/j.ijbiomac.2012.07.001
Capart R, Khezami L, Burnham AK (2004) Assessment of various kinetic models for the pyrolysis of a microgranular cellulose. Thermochim Acta 417:79–89. https://doi.org/10.1016/j.tca.2004.01.029
Šesták J (2017) Šesták-Berggren equation: Now questioned but formerly celebrated - what is right. J Therm Anal Calorim 127:1117–1123. https://doi.org/10.1007/s10973-015-4998-x
Ciolacu D, Popa VI (2006) On the Thermal degradation of cellulose allomorphs. Cellulose Chem Technol 40(6):445–449
Mukarakate C, Mital A, Ciesielski PN et al (2016) Influence of crystal allomorph and crystallinity on the products and behavior of cellulose during fast pyrolysis. ACS Sust Chem Eng 4(9):4662–4674. https://doi.org/10.1021/acssuschemeng.6b00812
Sang S, Zhuang X, Chen H et al (2022) Effect of supramolecular structural changes during the crystalline transformation of cellulose on its enzymatic hydrolysis. Ind Crop Prod 180:114687. https://doi.org/10.1016/j.indcrop.2022.114687
Wohlert M, Benselfelt T, Wågberg L et al (2022) Cellulose and the role of hydrogen bonds: not in charge of everything. Cellulose 29:1–23. https://doi.org/10.1007/s10570-021-04325-4
Pan M, Zhou X, Chen M (2013) Cellulose nanowhiskers isolation and properties from acid hydrolysis combined with high pressure homogenization. BioRes 8(1):933–943. https://doi.org/10.15376/biores.8.1.933-943
Sèbe G, Ham-Pichavant F, Ibarboure E, Koffi ALC, Tingaut P (2012) Supramolecular structure characterization of cellulose II nanowhiskers produced by acid hydrolysis of cellulose I substrates. Biomacromol 13(2):570–578. https://doi.org/10.1021/bm201777j
Beckham GT, Matthews JF, Peters B et al (2011) Molecular-level origins of biomass recalcitrance: Decrystallization free energies for four common cellulose polymorphs. J Phys Chem B 115(14):4118–4127. https://doi.org/10.1021/jp1106394
Mumbach GD, Alves JLF, Da Silva JCG et al (2019) Thermal investigation of plastic solid waste pyrolysis via the deconvolution technique using the asymmetric double sigmoidal function: determination of the kinetic triplet, thermodynamic parameters, thermal lifetime and pyrolytic oil composition for clean energy recovery. Energy Convers Manag 200:112031. https://doi.org/10.1016/j.enconman.2019.112031
Goldberg RN, Schliesser J, Mittal A et al (2015) A thermodynamic investigation of the cellulose allomorphs: cellulose(am), cellulose Iβ(cr), cellulose II(cr), and cellulose III(cr). J Chem Thermodyn 81:184–226. https://doi.org/10.1016/j.jct.2014.09.006
Ioelovich M (2021) Adjustment of hydrophobic properties of cellulose materials. Polymers 13(8):1241. https://doi.org/10.3390/polym13081241
Dalle-Ferrier C, Kisliuk A, Hong L et al (2016) Why many polymers are so fragile: A new perspective. J Chem Phys 145:154901. https://doi.org/10.1063/1.4964362
Huang D, McKenna GB (2001) New insights into the fragility dilemma in liquids. J Chem Phys 114:5621–5630. https://doi.org/10.1063/1.1348029
Liu C, Liu Z, Yin X, Wu G (2015) Tuning the dynamic fragility of acrylic polymers by small molecules: The interplay of hydrogen bonding strength. Macromolecules 48(12):4196–4206. https://doi.org/10.1021/acs.macromol.5b00489
Rachocki A, Markiewicz E, Tritt-Goc J (2005) Dielectric relaxation in cellulose and its derivatives. Acta Phys Pol Ser A 108(1):137–145. https://doi.org/10.12693/APhysPolA.108.137
Erdmann R, Kabasci S, Heim H-P (2021) Thermal properties of plasticized cellulose acetate and its β-relaxation phenomenon. Polymers 13(9):1356. https://doi.org/10.3390/polym13091356
Funding
Authors would like to acknowledge financial support of Ministry of Science, Technological Development and Innovation of the Republic of Serbia, under Contract numbers 451-03-47/2023-01/200017 (“Vinča” Institute of Nuclear Sciences—National Institute of the Republic of Serbia), 451-03-47/2023-01/200105 (Faculty of Mechanical Engineering), and 451-03-47/2023-01/200051 (Institute of General and Physical Chemistry).
Author information
Authors and Affiliations
Contributions
BJ was contributed to conceptualization, methodology, software, validation, formal analysis, data curation, writing—original draft, writing—review and editing, visualization, supervision, and project administration. MM-C was contributed to validation, investigation, data curation, writing—review and editing, and supervision. NM was contributed to methodology, software, validation, investigation, data curation, and supervision. MJ was contributed to methodology, validation, formal analysis, investigation, data curation, and supervision. HW was contributed to validation, formal analysis, investigation, resources, data curation, and supervision. VD was contributed to validation, investigation, resources, data curation, writing—review and editing, and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Human or animal rights
This research does not involve human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janković, B., Marinović-Cincović, M., Manić, N. et al. Enthalpy–entropy compensation and isoequilibrium relationship in thermo-chemical conversion of cigarette butt filters (CBFs) based on cellulose acetate (CA): causes and effects. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05343-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05343-5