Abstract
Using feather keratin (FK), a kind of biocompatible natural polymer being extracted from widely available and renewable waste feathers, and sodium alginate (SA) as materials, the FK conjugated SA gel beads (FK-SA-Gbs) were prepared with double cross-linked by calcium ion (Ca2+) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC). After being characterized and analyzed by Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), and X-ray diffraction (XRD), its pH responsiveness, salt ion responsiveness, and degradability were measured, and the loading capacity of model drug Rhodamine B (RhB) under various physical conditions, the cumulative release rate of the loaded gel beads in different pH buffer solutions were also investigated. The results showed that the FK-SA-Gbs had significant pH sensitivity and sustained release, and various physical conditions can meet the requirements of wound dressings. It indicated FK-SA-Gbs can be used as controlled release drug carriers and are expected to be used as drug carrier materials in biomedical applications.
Graphical abstract
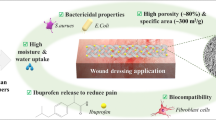
FK conjugated SA gel beads (FK-SA-Gbs) was prepared by secondary cross-linking with CaCl2 and 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride (EDC/HCl) using feather keratin (FK) extracted from waste feathers, which have significant pH sensitivity and sustained release capability.











Similar content being viewed by others
References
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A (2014) Cancer treatment and survivorship statistics. CA Cancer J Clin 64(4):252–271. https://doi.org/10.3322/caac.21235
Hubbell JA, Langer R (2013) Translating materials design to the clinic. Nat Mater 12(11):963–966. https://doi.org/10.1038/nmat3788
Elsabahy M, Wooley KL (2012) Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev 41(7):2545–2561. https://doi.org/10.1039/C2CS15327K
Sun K, Guo J, He Y, Song P, **ong Y, Wang RM (2016) Fabrication of dual-sensitive keratin-based polymer hydrogels and their controllable release behaviors. J Biomat Sci Polym Ed 27(18):1926–1940. https://doi.org/10.1080/09205063.2016.1239955
Jiang T, Mo R, Bellotti A, Zhou J, Gu Z (2014) Gel-liposome-mediated co-delivery of anticancer membrane-associated proteins and small-molecule drugs for enhanced therapeutic efficacy. Adv Funct Mater 24(16):2295–2304. https://doi.org/10.1002/adfm.201303222
Li X, Gao F, Dong Y, Li X (2019) Strategies to regulate the degradability of mesoporoussilica-based nanoparticles for biomedical applications. Nano 14(12):1930008. https://doi.org/10.1142/S1793292019300081
Xu R, Zhang G, Mai J, Deng X, Segura-Ibarra V, Wu S, Shen J, Liu H, Hu Z, Chen L, Huang Y, Koay E, Huang Y, Liu J, Ensor JE, Blanco E, Liu X, Ferrari M, Shen H (2016) An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol 34(4):414–418. https://doi.org/10.1038/nbt.3506
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12(11):991–1003. https://doi.org/10.1038/NMAT3776
Petros RA, DeSimone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9(8):615–627. https://doi.org/10.1038/nrd2591
Fang R, Yang S, Wang Y, Qian H (2015) Nanoscale drug delivery systems: A current review on the promising paclitaxel formulations for future cancer therapy. Nano 10(5):1530004. https://doi.org/10.1142/S1793292015300042
Cai K, He X, Song Z, Yin Q, Zhang Y, Uckun FM, Jiang C, Cheng J (2015) Dimeric drug polymeric nanoparticles with exceptionally high drug loading and quantitative loading efficiency. J Am Chem Soc 137(10):3458–3461. https://doi.org/10.1021/ja513034e
Hubbell JA, Chilkoti A (2012) Nanomaterials for drug delivery. Science 337(6092):303–305. https://doi.org/10.1126/science.1219657
Chung JE, Tan S, Gao SJ, Yongvongsoontorn N, Kim SH, Lee JH, Choi HS, Yano H, Zhuo L, Kurisawa M, Ying JY (2014) Self-assembled micellar nanocomplexes comprising greentea catechin derivatives and protein drugs for cancer therapy. Nat Nano 9(11):907–912. https://doi.org/10.1038/nnano.2014.208
Li XM, Wang B, He YF, Song P, Yan G, Wang R (2021) Soybean protein isolate-based microgels bounding amino acid metal complexes for scavenging superoxide anion radicals. Polym Bull 78(2):713–728. https://doi.org/10.1007/s00289-020-03121-7
Stergar J, Maver U (2016) Review of aerogel-based materials in biomedical applications. J Sol–Gel Sci Techn 77(3):738–752. https://doi.org/10.1007/s10971-016-3968-5
Franke-Whittle IH, Insam H (2013) Treatment alternatives of slaughterhouse wastes, and their effect on the inactivation of different pathogens: a review. Crit Rev Microbiol 39(2):139–151. https://doi.org/10.3109/1040841X.2012.694410
Li M, Zhu Z, Pan X (2011) Effects of starch acryloylation on the grafting efficiency, adhesion, and film properties of acryloylated starch-g-poly (acrylic acid) for warp sizing. Starch-Starke 63(11):683–691. https://doi.org/10.1002/star.201100002
Kucinska JK, Magnucka EG, Oksinska MP, Pietr SJ (2014) Bio efficacy of hen feather keratin hydrolysate and compost on vegetable plant growth. Compost Sci Util 22(3):179–187. https://doi.org/10.1080/1065657X.2014.918866
Sadeghi S, Dadashian F, Eslahi N (2019) Recycling chicken feathers to produce adsorbent porous keratin-based sponge. Int J Environ Sci Technol 16(2):1119–1128. https://doi.org/10.1007/s13762-018-1669-z
Nakata R, Osumi Y, Miyagawa S, Tachibana A, Tanabe T (2015) Preparation of keratin andchemically modified keratin hydrogels and their evaluation as cell substrate with drug releasing ability. J Biosci Bioeng 120(1):111–116. https://doi.org/10.1016/j.jbiosc.2014.12.005
Gao L, Li R, Sui X, Li R, Chen C, Chen Q (2014) Conversion of chicken feather waste toN-doped carbon nanotubes for the catalytic reduction of 4-nitrophenol. Environ Sci Technol 48(17):10191–10197. https://doi.org/10.1021/es5021839
Pardo-Ibáñez P, Lopez-Rubio A, Martínez-Sanz M, Cabedo L, Lagaron JM (2014) Keratin–polyhydroxyalkanoate melt-compounded composites with improved barrier properties of interest in food packaging applications. J Appl Polym Sci. https://doi.org/10.1002/app.39947
Yin XC, Li FY, He YF, Wang Y, Wang RM (2013) Study on effective extraction of chicken feather keratins and their films for controlling drug release. Biomater Sci 1(5):528–536. https://doi.org/10.1039/C3BM00158J
**a W, **e M, Feng X, Chen L, Zhao Y (2018) Surface modification of poly (vinylidene fluoride) ultrafiltration membranes with chitosan for anti-fouling and antibacterial performance. Macromol Res 26(13):1225–1232. https://doi.org/10.1007/s13233-019-7019-2
Feiz S, Navarchian AH (2019) Poly (vinyl alcohol) hydrogel/chitosan-modified clay nanocomposites for wound dressing application and controlled drug release. Macromol Res 27(3):290–300. https://doi.org/10.1007/s13233-019-7046-z
Xue Y, **a X, Yu B, Luo X, Cai N, Long S, Yu F (2015) A green and facile method forthe preparation of a pH-responsive alginate nanogel for subcellular delivery of doxorubicin. RSC Adv 5(90):73416–73423. https://doi.org/10.1039/C5RA13313K
Alonso S (2018) Exploiting the bioengineering versatility of lactobionic acid in targeted nano systems and biomaterials. J Control Release 287:216–234. https://doi.org/10.1016/j.jconrel.2018.08.030
Sarika PR, James NR (2016) Polyelectrolyte complex nanoparticles from cationised gelatin and sodium alginate for curcumin delivery. Carbohyd Polym 148:354–361. https://doi.org/10.1016/j.carbpol.2016.04.073
Tam SK, Dusseault J, Polizu S, Ménard M, Hallé JP, Yahia LH (2005) Physicochemical model of alginate-poly-l-lysine microcapsules defined at the micrometric/nanometric scale using ATR-FTIR, XPS, and ToF-SIMS. Biomaterials 26(34):6950–6961. https://doi.org/10.1016/j.biomaterials.2005.05.007
Acknowledgements
The project was supported by the National Natural Science Foundation of China (Grant No. 21865030).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Ren, J., Qian, L. et al. Preparation of FK-SA conjugate gel beads with double cross-linking for pH-controllable drug releasing. Polym. Bull. 80, 331–347 (2023). https://doi.org/10.1007/s00289-022-04076-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04076-7