Summary
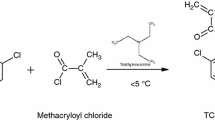
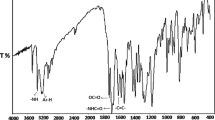
The methacrylic monomer, 4-biphenylmethacrylate (BPM) was synthesized by reacting 4-biphenyl phenol dissolved in ethyl methyl ketone (EMK) with methacryloyl chloride in presence of triethylamine as a catalyst. The copolymers of BPM with glycidyl methacrylate (GMA) were synthesized by free radical polymerization in EMK solution at 70±1 °C using benzoyl peroxide as a free radical initiator. The copolymerization behaviour was studied in a wide composition interval with the mole fractions of BPM ranging from 0.15 to 0.9 in the feed. The copolymers were characterized by FT-IR, 1H-NMR and 13C-NMR spectroscopic techniques. The solubility was tested in various polar and non polar solvents. The molecular weight and polydispersity indices of the polymers were determined using gel permeation chromatography. The glass transition temperature of the copolymers increases with increase in BPM content. The thermogravimetric analysis of the polymers showed that the thermal stability of the copolymer increases with BPM content. The copolymer composition was determined using 1H-NMR spectra. The monomer reactivity ratios were determined by the application of conventional linearization methods such as Fineman-Ross (r1=0.392 ± 0.006, r2 = 0.358 ± 0.007, Kelen-Tudos (r1= 0.398 ± 0.004, r2= 0.365 ± 0.013) and extended Kelen-Tudos methods (r1= 0.394 ± 0.004, r2= 0.352 ± 0.006).
Similar content being viewed by others
References
Manesh DBD, Reddy BSR, Arshady R, George MH (1985) Polymer 27: 96.
Vijayanand PS, Penlidis A, Nanjundan S (2003) J Macro Sci-Pure Appl Chem 40(2): 125.
Soykan C, Ahmedzade M, Coskun M (2000) Eur Polym J 36(8): 1667.
Balasubramanian S, Reddy BSR (1996) Eur Polym J 32(9): 1073.
Pitchumani S, Rami Reddy AV, Rajadurai S (1982) J Polym Sci Polym Chem Edn 20: 277.
Bankova M, Petrova TS, Manolova N, Rashkov I (1996) Eur Polym J 2(5): 569.
Reis AV, Cavalcanti OA, Rubira AF, Muniz EC (2003) Int J Pharm 267: 13.
Ghosh S, Krishnamurthi N (2000) Euro Polym J 36(10): 2125.
Labella R, Braden M, Davy KWM (1992) Biomaterials 13(13): 937.
Guilherme MR, Reis AV, Takahashi SH, Rubira AF, Feitosa JPA, Muniz EC (2005). Carbohydrate Polymers 61(4): 464.
Monnereau C, Blart E, Montembault Fontaine VL, Odobel F (2005) Tetrahedron 61(42): 10113.
Hua JL, Yip Lam JW, Dong H, Wu L, Wong KS, Tang BZ (2006) Polymer 47(1).
Ichimura K, Nishio Y (1987) J Polym Sci Part A Poly Chem 25: 1579.
Vijayanand PS, Arun Prasath R, Balaji R, Nanjundan S (2002) J Appl Polym Sci 85(11): 2261.
Vijayaraghavan PG, Reddy BSR (1996) J Appl Polym Sci 61(6): 936.
Jone Selvamalar CS, Vijayanand PS, Penlidis A, Nanjundan S (2004) J Appl Polym Sci 91: 3604.
Balaji R, Grande D, Nanjundan S (2004) Polymer 45(4): 1089.
Pandey SC, Rather N, Singh A (1999) J Polym Mat 16(3): 253.
Arun A, Reddy BSR (2005) Biomaterials 26(10): 1185.
Johnck M, Muller L, Neyer A, Hofstraat JW (2000) Eur Polym J 36(6): 1251.
Yarapathi RV, Kurva, Tamishetti S (2004) Cat Comm 5(9): 511.
Vijayanand PS, Penlidis A, Radhakrishnan S, Nanjundan S(2002) J Mac Sci-Pure Appl Chem 39(6): 591.
Soundarajan S, Reddy BSR (1991) J Appl Polym Sci 43(2): 251.
Vijayanand PS, Jone Selvamalar CS, Penlidis A, Nanjundan S (2003) Polym Int 52(12): 1856.
Gatica N, Gargallo L, Radic D (2002) Eur Polym J 38(7): 1371.
Thamizharasi S, Reddy BSR (2001) J Appl Polym Sci 80: 1870.
Stampel G.H, Cross RP, Mariella RP (1950) J Am Chem Soc 72: 2299.
Alberda Van Ekenstein G.OR, Altena HJH, Tan YY (1989) Eur Polym J 25(2): 111.
Teramachi S, Hasegawa A, Atasuka M, Yamashita A, Takemoto N (1978) Macromolecules 11: 1206.
Melville H.W, Noble B, Watson WF (1949) J Polym Sci 4(5): 629.
Thamizharasi S, Reddy BSR (2000) Eur Polym J 36: 993.
Fineman M, Ross SD (1950) J Polym Sci 5(2): 259.
Kelen T, Tudos F (1975) J Mac Sci Pure & Appl Chem A9: 1.
Kelen T, Tudos F, Turesanyi B, Kennedy JP (1977) J Polym Sci Poly Chem 15: 3041.
Narasimhaswamy T, Sumathi SC, Reddy BSR (1991) Polymer 32(18): 3427.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vijayanand, P., Kato, S., Satokawa, S. et al. Copolymerization of 4-biphenyl methacrylate with glycidyl methacrylate: Synthesis, Characterization, thermal properties and determination of monomer reactivity ratios. Polym. Bull. 58, 861–872 (2007). https://doi.org/10.1007/s00289-007-0728-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-007-0728-2