Abstract
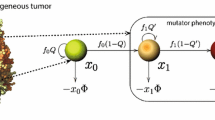
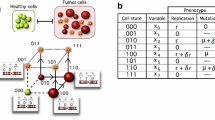
Cancer is usually considered a genetic disease caused by alterations in genes that control cellular behaviors, especially growth and division. Cancer cells differ from normal tissue cells in many ways that allow them to grow out of control and become invasive. However, experiments have shown that aberrant growth in many tissues burdened with varying numbers of mutant cells can be corrected, and wild-type cells are required for the active elimination of mutant cells. These findings reveal the dynamic cellular behaviors that lead to a tissue homeostatic state when faced with mutational and nonmutational insults. The current study was motivated by these observations and established a mathematical model of how a tissue copes with the aberrant behavior of mutant cells. The proposed model depicts the interaction between wild-type and mutant cells through a system of two delay differential equations, which include the random mutation of normal cells and the active extrusion of mutant cells. Based on the proposed model, we performed qualitative analysis to identify the conditions of either normal tissue homeostasis or uncontrolled growth with varying numbers of abnormal mutant cells. Bifurcation analysis suggests the conditions of bistability with either a small or large number of mutant cells, the coexistence of bistable steady states can be clinically beneficial by driving the state of mutant cell predominance to the attraction basin of the state with a low number of mutant cells. This result is further confirmed by the treatment strategy obtained from optimal control theory.









Similar content being viewed by others
References
Altrock PM, Liu LL, Michor F (2015) The mathematics of cancer: integrating quantitative models. Nat Rev Cancer 15(12):730–745
Anderson ARA (2005) A hybrid mathematical model of solid tumour invasion: the importance of cell adhesion. Math Med Biol 22(2):163–186
Anderson ARA, Weaver AM, Cummings PT, Quaranta V (2006) Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell 127(5):905–915
Araujo RP, Mcelwain DLS (2004) A history of the study of solid tumour growth: the contribution of mathematical modelling. Bull Math Biol 66(5):1039–1091
Beretta E, Kuang Y (2002) Geometric stability switch criteria in delay differential systems with delay dependent parameters. SIAM J Math Anal 33(5):1144–1165
Bernard S, Bélair J, Mackey MC (2003) Oscillations in cyclical neutropenia: new evidence based on mathematical modeling. J Theor Biol 223(3):283–298
Bissell MJ, Hines WC (2011) Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17(3):320–329
Brown S, Pineda CM, **n T, Boucher J, Suozzi KC, Park S, Matte-Martone C, Gonzalez DG, Rytlewski J, Beronja S, Greco V (2017) Correction of aberrant growth preserves tissue homeostasis. Nature 548(7667):334–337
Brunetti M, Mackey MC, Craig M (2021) Understanding normal and pathological hematopoietic stem cell biology using mathematical modelling. Curr Stem Cell Rep 7(3):109–120
Burns FJ, Tannock IF (1970) On the existence of a \(G_0\)-phase in the cell cycle. Cell Tiss Kinet 3(4):321–334
Castiglione M, Zhang H, Kaushansky K, Zhan H (2021) Cell competition between wild-type and JAK2V617F mutant cells in a murine model of a myeloproliferative neoplasm. Exp Hematol 100:52–62
Celso CL, Prowse DM, Watt FM (2004) Transient activation of \(\beta \)-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131(8):1787–1799
Chen CL, Schroeder MC, Kango-Singh M, Tao C, Halder G (2012) Tumor suppression by cell competition through regulation of the Hippo pathway. Proc Natl Acad Sci USA 109(2):484–489
Colijn C, Mackey MC (2005) A mathematical model of hematopoiesis-I. Periodic chronic myelogenous leukemia. J Theor Biol 237(2):117–132
Colom B, Herms A, Hall MWJ, Dentro SC, King C, Sood RK, Alcolea MP, Piedrafita G, Fernandez-Antoran D, Ong SH, Fowler JC, Mahbubani KT, Saeb-Parsy K, Gerstung M, Hall BA, Jones PH (2021) Mutant clones in normal epithelium outcompete and eliminate emerging tumours. Nature 598(7881):510–514
Craig M, Humphries AR, Nekka F, Bélair J, Li J, Mackey MC (2015) Neutrophil dynamics during concurrent chemotherapy and G-CSF administration: mathematical modelling guides dose optimisation to minimise neutropenia. J Theor Biol 385:77–89
De Souza DC, Humphries AR (2019) Dynamics of a mathematical hematopoietic stem-cell population model. SIAM J Appl Dyn Syst 18(2):808–852
Gatenby RA, Vincent TL (2003) An evolutionary model of carcinogenesis. Cancer Res 63(19):6212–6220
Ghaffarizadeh A, Heiland R, Friedman SH, Mumenthaler SM, Macklin P (2018) PhysiCell: an open source physics-based cell simulator for 3-D multicellular systems. PLoS Comput Biol 14(2):e1005991
Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, Edkins S, O’Meara S, Vastrik I, Schmidt EE, Avis T, Barthorpe S, Bhamra G, Buck G, Choudhury B, Clements J, Cole J, Dicks E, Forbes S, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jenkinson A, Jones D, Menzies A, Mironenko T, Perry J, Raine K, Richardson D, Shepherd R, Small A, Tofts C, Varian J, Webb T, West S, Widaa S, Yates A, Cahill DP, Louis DN, Goldstraw P, Nicholson AG, Brasseur F, Looijenga L, Weber BL, Chiew YE, deFazio A, Greaves MF, Green AR, Campbell P, Birney E, Easton DF, Chenevix-Trench G, Tan MH, Khoo SK, Teh BT, Yuen ST, Leung SY, Wooster R, Futreal PA, Stratton MR (2007) Patterns of somatic mutation in human cancer genomes. Nature 446(7132):153–158
Hale JK, Verduyn Lunel SM (1993) Introduction to functional differential equations. Springer-Verlag, New York
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Hayes ND (1950) Roots of the transcendental equation associated with a certain difference-differential equation. J Lond Math Soc 25(3):226–232
Hill W, Zaragkoulias A, Salvador-Barbero B, Parfitt GJ, Alatsatianos M, Padilha A, Porazinski S, Woolley TE, Morton JP, Sansom OJ, Hogan C (2021) EPHA2-dependent outcompetition of KRASG12D mutant cells by wild-type neighbors in the adult pancreas. Curr Biol 31(12):2550–2560
Hogan C, Dupré-Crochet S, Norman M, Kajita M, Zimmermann C, Pelling AE, Piddini E, Baena-López LA, Vincent JP, Itoh Y, Hosoya H, Pichaud F, Fujita Y (2009) Characterization of the interface between normal and transformed epithelial cells. Nat Cell Biol 11(4):460–467
Hogan C, Kajita M, Lawrenson K, Fujita Y (2011) Interactions between normal and transformed epithelial cells: their contributions to tumourigenesis. Int J Biochem Cell Biol 43(4):496–503
Hu M, Polyak K (2008) Microenvironmental regulation of cancer development. Curr Opin Genet Dev 18(1):27–34
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9(4):239–252
Kajita M, Hogan C, Harris AR, Dupre-Crochet S, Itasaki N, Kawakami K, Charras G, Tada M, Fujita Y (2010) Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J Cell Sci 123(2):171–180
Kon S, Ishibashi K, Katoh H, Kitamoto S, Shirai T, Tanaka S, Kajita M, Ishikawa S, Yamauchi H, Yako Y, Kamasaki T, Matsumoto T, Watanabe H, Egami R, Sasaki A, Nishikawa A, Kameda I, Maruyama T, Narumi R, Morita T, Sasaki Y, Enoki R, Honma S, Imamura H, Oshima M, Soga T, Miyazaki JI, Duchen MR, Nam JM, Onodera Y, Yoshioka S, Kikuta J, Ishii M, Imajo M, Nishida E, Fujioka Y, Ohba Y, Sato T, Fujita Y (2017) Cell competition with normal epithelial cells promotes apical extrusion of transformed cells through metabolic changes. Nat Cell Biol 19(5):530–541
Lei J (2020) A general mathematical framework for understanding the behavior of heterogeneous stem cell regeneration. J Theor Biol 492:110196
Lei J (2020) Evolutionary dynamics of cancer: from epigenetic regulation to cell population dynamics-mathematical model framework, applications, and open problems. Sci China Math 63(3):411–424
Lei J, Mackey MC (2007) Stochastic differential delay equation, moment stability, and application to hematopoietic stem cell regulation system. SIAM J Appl Math 67(2):387–407
Lenhart S, Workman JT (2007) Optimal control applied to biological models. Chapman & Hall/CRC, London
Li Q, Bohin N, Wen T, Ng V, Magee J, Chen SC, Shannon K, Morrison SJ (2013) Oncogenic nras has bimodal effects on stem cells that sustainably increase competitiveness. Nature 504(7478):143–147
Lowengrub JS, Frieboes HB, ** F, Chuang YL, Li X, Macklin P, Wise SM, Cristini V (2010) Nonlinear modelling of cancer: bridging the gap between cells and tumours. Nonlinearity 23(1):R1–R91
Mackey MC (1978) Unified hypothesis for the origin of aplastic anemia and periodic hematopoiesis. Blood 51(5):941–956
Mackey MC (2001) Cell kinetic status of haematopoietic stem cells. Cell Prolif 34(2):71–83
Martins VC, Busch K, Juraeva D, Blum C, Ludwig C, Rasche V, Lasitschka F, Mastitsky SE, Brors B, Hielscher T, Fehling HJ, Rodewald HR (2014) Cell competition is a tumour suppressor mechanism in the thymus. Nature 509(7501):465–470
Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J (2010) Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol 8(3):e1000324
Morata G, Ripoll P (1975) Minutes: mutants of drosophila autonomously affecting cell division rate. Dev Biol 42(2):211–221
Moreno E, Basler K (2004) dMyc transforms cells into super-competitors. Cell 117(1):117–129
Moustakas A, Pardali K, Gaal A, Heldin CH (2002) Mechanisms of TGF-\(\beta \) signaling in regulation of cell growth and differentiation. Immunol Lett 82(1–2):85–91
Moya IM, Castaldo SA, Van den Mooter L, Soheily S, Sansores-Garcia L, Jacobs J, Mannaerts I, **e J, Verboven E, Hillen H, Algueró-Nadal A, Karaman R, Van Haele M, Kowalczyk W, De Waegeneer M, Verhulst S, Karras P, van Huffel L, Zender L, Marine JC, Roskams T, Johnson R, Aerts S, van Grunsven LA, Halder G (2019) Peritumoral activation of the Hippo pathway effectors YAP and TAZ suppresses liver cancer in mice. Science 366(6468):1029–1034
Parker TM, Gupta K, Palma AM, Yekelchyk M, Fisher PB, Grossman SR, Won KJ, Madan E, Moreno E, Gogna R (2021) Cell competition in intratumoral and tumor microenvironment interactions. EMBO J 40(17):e107271
Polyak K, Haviv I, Campbell IG (2009) Co-evolution of tumor cells and their microenvironment. Trends Genet 25(1):30–38
Pontryagin LS, Boltyanskii VG, Gamkrelidze RV, Mishchenko EF (1962) The mathematical theory of optimal processes. Interscience Publishers, New York
Pujo-Menjouet L, Bernard S, Mackey MC (2005) Long period oscillations in a \(G_0\) model of hematopoietic stem cells. SIAM J Appl Dyn Syst 4(2):312–332
Pujo-Menjouet L, Mackey MC (2004) Contribution to the study of periodic chronic myelogenous leukemia. C R Biol 327(3):235–244
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19(11):1423–1437
Ramos CV, Martins VC (2021) Cell competition in hematopoietic cells: quality control in homeostasis and its role in leukemia. Dev Biol 475:1–9
Rocha HL, Almeida RC, Lima EABF, Resende ACM, Oden JT, Yankeelov TE (2018) A hybrid three-scale model of tumor growth. Math Models Meth Appl Sci 28(1):61–93
Sun Q, Luo T, Ren Y, Florey O, Shirasawa S, Sasazuki T, Robinson DN, Overholtzer M (2014) Competition between human cells by entosis. Cell Res 24(11):1299–1310
Van Egeren D, Escabi J, Nguyen M, Liu S, Reilly CR, Patel S, Kamaz B, Kalyva M, DeAngelo DJ, Galinsky I, Wadleigh M, Winer ES, Luskin MR, Stone RM, Garcia JS, Hobbs GS, Camargo FD, Michor F, Mullally A, Cortes-Ciriano I, Hormoz S (2021) Reconstructing the lineage histories and differentiation trajectories of individual cancer cells in myeloproliferative neoplasms. Cell Stem Cell 28(3):514–523
van Neerven SM, de Groot NE, Nijman LE, Scicluna BP, van Driel MS, Lecca MC, Warmerdam DO, Kakkar V, Moreno LF, Braga FAV, Sanches DR, Ramesh P, ten Hoorn S, Aelvoet AS, van Boxelk MF, Koens L, Krawczyk PM, Koster J, Dekker E, Medema JP, Winton DJ, Bijlsma MF, Morrissey E, Léveillé N, Vermeulen L (2021) Apc-mutant cells act as supercompetitors in intestinal tumour initiation. Nature 594(7863):436–441
Vishwakarma M, Piddini E (2020) Outcompeting cancer. Nat Rev Cancer 20(3):187–198
Vogelstein B, Kinzler KW (2004) Cancer genes and the pathways they control. Nat Med 10(8):789–799
Yamamoto M, Ohsawa S, Kunimasa K, Igaki T (2017) The ligand sas and its receptor PTP10D drive tumour-suppressive cell competition. Nature 542(7640):246–250
Yang H, Lei J (2019) A mathematical model of chromosome recombination-induced drug resistance in cancer therapy. Math Biosci Eng 16(6):7098–7111
Yang L, Pang Y, Moses HL (2010) TGF-\(\beta \) and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 31(6):220–227
Zhang J, Cunningham JJ, Brown JS, Gatenby RA (2017) Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat Commun 8:1816
Acknowledgements
This work was funded by the National Natural Science Foundation of China (NSFC 11831015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Zhang, M. & Lei, J. A mathematical model with aberrant growth correction in tissue homeostasis and tumor cell growth. J. Math. Biol. 86, 2 (2023). https://doi.org/10.1007/s00285-022-01837-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-022-01837-w