Abstract
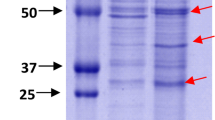
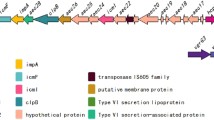
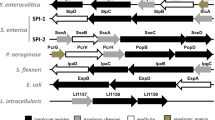
Helicobacter pullorum is a human zoonotic pathogen transmitted through poultry where it is associated with vibrionic hepatitis and colitis. Hemolysin co-regulated protein (Hcp) is an important structural as well as effector protein of type six secretory system; however, its role in H. pullorum invasion and pathogenesis has not been elucidated. In this study, we predicted the Helicobacter pullorum Hcp (HpuHcp) structure and identified Campylobacter jejuni Hcp (CjHcp) as its nearest homologue. Analysis of the predicted structure shows several common bacterial Hcp motifs like Protein kinase C phosphorylation site, Casein kinase II phosphorylation site, N-myristoylation site, cAMP-and cCGMP-dependent protein kinase phosphorylation site, N-glycosylation site. The presence of unique microbodies C-terminal targeting signal domain was present in HpuHcp which was seen for the first time in CjHcp. This could indicate that Hcp is a structural protein as well as a secretory protein. Moreover, the presence of a deamidase domain, similar to the tecA of Burkholderia cenocepacia an opportunistic pathogen, may help in bacterial internalization as it depolymerises the membranous actin by deamidation of the host cell Rho GTPases cdc42 and Rac1, which was supported by increased invasion of hepatocytes by Hcp-positive isolates.





Similar content being viewed by others
References
Boyer F et al (2009) Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics 10:104
Ho BT, Dong TG, Mekalanos JJ (2014) A view to a kill: the bacterial type VI secretion system. Cell Host Microbe 15(1):9–21
Singh A, Mallick AI (2019) Role of putative virulence traits of Campylobacter jejuni in regulating differential host immune responses. J Microbiol 57(4):298–309
Logan SL (2018) Proc Natl Acad Sci USA 115(16): E3779-E3787
Borges V et al (2015) Helicobacter pullorum isolated from fresh chicken meat: antibiotic resistance and genomic traits of an emerging foodborne pathogen. Appl Environ Microbiol 81(23):8155–8163
Kaakoush NO et al (2014) The interplay between Campylobacter and Helicobacter species and other gastrointestinal microbiota of commercial broiler chickens. Gut Pathog 6:18
Sirianni A et al (2013) The pathogenic potential of Helicobacter pullorum: possible role for the type VI secretion system. Helicobacter 18(2):102–111
Lertpiriyapong K et al (2012) Campylobacter jejuni type VI secretion system: roles in adaptation to deoxycholic acid, host cell adherence, invasion, and in vivo colonization. PLoS ONE 7(8):e42842
Cascales E, Cambillau C (2012) Structural biology of type VI secretion systems. Philos Trans R Soc Lond B Biol Sci 367(1592):1102–1111
Records AR (2011) The type VI secretion system: a multipurpose delivery system with a phage-like machinery. Mol Plant Microbe Interact 24(7):751–757
Javed K et al (2019) Prevalence and role of Type six secretion system in pathogenesis of emerging zoonotic pathogen. Avian Pathol 1–7
Burnens AP et al (1994) Gastroenteritis associated with Helicobacter pullorum. Lancet 344(8936):1569–1570
Chen JE, Huang CC, Ferrin TE (2015) RRDistMaps: a UCSF Chimera tool for viewing and comparing protein distance maps. Bioinformatics 31(9):1484–1486
El-Housseiny GS, Aboulwafa MM, Hassouna NA (2010) Adherence, invasion and cytotoxicity of some bacterial pathogens. J of Am Sci 6(10):260–268
Silverman JM et al (2013) Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol Cell 51(5):584–593
Bleumink-Pluym NM et al (2013) Identification of a functional type VI secretion system in Campylobacter jejuni conferring capsule polysaccharide sensitive cytotoxicity. PLoS Pathog 9(5):e1003393
Coulthurst S (2019) The Type VI secretion system: a versatile bacterial weapon. Microbiology 165(5):503–515
Udenwobele DI et al (2017) Myristoylation: an important protein modification in the immune response. Front Immunol 8:751
Noreen Z et al (2018) Structural basis for the pathogenesis of Campylobacter jejuni Hcp1, a structural and effector protein of the Type VI Secretion System. FEBS J 285(21):4060–4070
Ham H, Sreelatha A, Orth K (2011) Manipulation of host membranes by bacterial effectors. Nat Rev Microbiol 9(9):635–646
MacIntyre DL et al (2010) The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci USA 107(45):19520–19524
Flannagan RS et al (2012) Burkholderia cenocepacia disrupts host cell actin cytoskeleton by inactivating Rac and Cdc42. Cell Microbiol 14(2):239–254
Sens P, Plastino J (2015) Membrane tension and cytoskeleton organization in cell motility. J Phys Condens Matter 27(27):273103
Tang DD, Gerlach BD (2017) The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir Res 18(1):54
Small JV et al (2002) The lamellipodium: where motility begins. Trends Cell Biol 12(3):112–120
Krause-Gruszczynska M et al (2007) Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell Microbiol 9(10):2431–2444
Nobes CD, Hall A (1995) Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81(1):53–62
Kaakoush NO et al (2011) The pathogenic potential of Campylobacter concisus strains associated with chronic intestinal diseases. PLoS ONE 6(12):e29045
Oelschlaeger TA, Guerry P, Kopecko DJ (1993) Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA 90(14):6884–6888
Funding
The research work described in this study was supported by the HEC-NRPU fund, Grant No. 7902/Federal/NRPU/R&D/HEC/2017.
Author information
Authors and Affiliations
Contributions
KJ contributed to experimental design, experimental work, in silico analysis and initial drafting of manuscript. FG collected samples and performed experimental work. SB contributed to the in silico analysis, writing and editing of the manuscript. HB and ZN contributed to initial concept, editing of the manuscript. RA contributed in the experimental design and experimental work. SJ contributed to the experimental design, writing and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest for this study.
Ethical approval
Ethical approval for the study for research involving animals for obtaining biological material (bacterial caecal isolates and data) was sought through the COMSATS Institute for Information Technology ethics review board (CIIT ERB), letter number CIIT/Bio/ERB/16/47.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
284_2022_2892_MOESM1_ESM.pdf
Supplementary figure 1: Structure of HpuHcp, indicating structural amino acids in yellow barrels with red labels and cyan barrels with black labels are those amino acids which are functional in nature. Table explaining the nature of residues as structural or functional (PDF 65 kb)
Rights and permissions
About this article
Cite this article
Javed, K., Gul, F., Abbasi, R. et al. In Silico and In Vitro Analysis of Helicobacter pullorum Type Six Secretory Protein Hcp and Its Role in Bacterial Invasion and Pathogenesis. Curr Microbiol 79, 195 (2022). https://doi.org/10.1007/s00284-022-02892-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02892-8