Abstract
Purpose
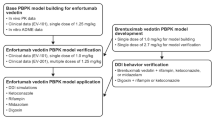
Entrectinib (ENT) is a potent c-ros oncogene 1(ROS1) and neurotrophic tyrosine receptor kinase (NTRKA/B/C) inhibitor. To determine the optimum dosage of ENT using ROS1 and NTRKA/B/C occupancy in plasma and cerebrospinal fluid (CSF) in drug–drug interactions (DDIs), physiologically-based pharmacokinetic (PBPK) models for healthy subjects and cancer population were developed for ENT and M5 (active metabolite).
Methods
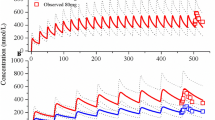
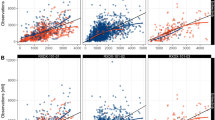
The PBPK models were built using the modeling parameters of ENT and M5 that were mainly derived from the published paper on the ENT PBPK model, and then validated by the observed pharmacokinetics (PK) in plasma and CSF from healthy subjects and patients.
Results
The PBPK model showed that AUC, Cmax, and Ctrough ratios between predictions and observations are within the range of 0.5–2.0, except that the M5 AUC ratio is slightly above 2.0 (2.34). Based on the efficacy (> 75% occupancy for ROS1 and NTRKA/B/C) and safety (AUC < 160 μM·h and Cmax < 8.9 μM), the appropriate dosing regimens were identified. The appropriate dosage is 600 mg once daily (OD) when administered alone, reduced to 200 mg and 400 mg OD with itraconazole and fluconazole, respectively. ENT is not recommended for co-administration with rifampicin or efavirenz, but is permitted with fluvoxamine or dexamethasone.
Conclusion
The PBPK models can serve as a powerful approach to predict ENT concentration as well as ROS1 and NTRKA/B/C occupancy in plasma and CSF.




Similar content being viewed by others
Data availability
Data are contained within the article or supplementary material.
References
Thandra KC, Barsouk A, Saginala K et al (2021) Epidemiology of lung cancer. Contemp Oncol 25(1):45–52
Huang Y, Zhang LJCI (2023) Annual progress of clinical research on targeted therapy for nonsmall cell lung cancer in 2022. Cancer Innovation 2(1):25–35
Coleman N, Hong L, Zhang J et al (2021) Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer. ESMO Open 6(6):100319
Farago AF, Taylor MS, Doebele RC et al (2018) Clinicopathologic features of non–small-cell lung cancer harboring an NTRK gene fusion. JCO Precis Oncol 2:1–12
Demetri GD, De Braud F, Drilon A et al (2022) Updated integrated analysis of the efficacy and safety of entrectinib in patients with NTRK fusion-positive solid tumors. Clin Cancer Res.
Gaebe K, Li AY, Das SJC (2021) Clinical biomarkers for early identification of patients with intracranial metastatic disease. Cancers (Basel) 13(23):5973
Wang Y, Shen S, Hu P et al (2022) Alectinib versus crizotinib in ALK-positive advanced non-small-cell lung cancer and comparison of next-generation TKIs after crizotinib failure: Real-world evidence. Cancer Med 11(23):4491–4500
Al-Salama ZT, Keam SJJD (2019) Entrectinib: first global approval. Drugs 79(13):1477–1483
Delgado J, Pean E, Melchiorri D et al (2021) The European medicines agency review of entrectinib for the treatment of adult or paediatric patients with solid tumours who have a neurotrophic tyrosine receptor kinase gene fusions and adult patients with non-small-cell lung cancer harbouring ROS1 rearrangements. ESMO Open 6(2):100087
Dziadziuszko R, Hung T, Wang K et al (2022) Pre-and post-treatment blood-based genomic landscape of patients with ROS1 or NTRK fusion-positive solid tumours treated with entrectinib. Mol Oncol 16(10):2000–2014
Food and Drug Administration (FDA). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212725Orig1s000,%20212726Orig1s000MultidisciplineR.pdf (accessed on 1, May, 2023).
Georgi V, Schiele F, Berger B-T et al (2018) Binding kinetics survey of the drugged kinome. J Am Chem Soc 140(46):15774–15782
Xu L, Yu S, Liu H et al (2022) Physiologically based pharmacokinetic combined BTK occupancy modeling for optimal dosing regimen prediction of acalabrutinib in patients alone, with different CYP3A4 variants, co-administered with CYP3A4 modulators and with hepatic impairment. Eur J Clin Pharmacol 78(9):1435–1446
Yamazaki SJTAJ (2013) Translational pharmacokinetic-pharmacodynamic modeling from nonclinical to clinical development: a case study of anticancer drug, crizotinib. AAPS J 15:354–366
Yamamoto Y, Välitalo PA, Huntjens DR et al (2017) Predicting drug concentration-time profiles in multiple CNS compartments using a comprehensive physiologically-based pharmacokinetic model. CPT Pharmacometrics Syst Pharmacol 6(11):765–777
Adiwidjaja J, Gross AS, Boddy AV et al (2022) Physiologically-based pharmacokinetic model predictions of inter-ethnic differences in imatinib pharmacokinetics and dosing regimens. Br J Clin Pharmacol 88(4):1735–1750
Wang Z, Liu W, Li X et al (2022) Physiologically based pharmacokinetic combined JAK2 occupancy modeling to simulate PK and PD of baricitinib with kidney transporter inhibitors and in patients with hepatic/renal impairment. Regul Toxicol Pharmacol 133:105210
Alsmadi MtM, AL‐Daoud NM, Jaradat MM, et al (2021) Physiologically‐based pharmacokinetic model for alectinib, ruxolitinib, and panobinostat in the presence of cancer, renal impairment and hepatic impairment. Biopharm Drug Dispos 42 (6): 263-284
Djebli N, Buchheit V, Parrott N et al (2021) Physiologically-based pharmacokinetic modeling of entrectinib parent and active metabolite to support regulatory decision-making. Eur J Drug Metab Pharmacokinet 46:779–791
Pharmaceuticals and Medical Devices Agency (PMDA). Available online: https://www.info.pmda.go.jp/go/interview/1/450045_4291061M1025_1_008_1F.pdf (accessed on 1, May, 2023).
Parrott N, Stillhart C, Lindenberg M et al (2020) Physiologically based absorption modelling to explore the impact of food and gastric pH changes on the pharmacokinetics of entrectinib. AAPS J 22:1–13
Menichincheri M, Ardini E, Magnaghi P et al (2016) Discovery of entrectinib: a new 3-aminoindazole as a potent anaplastic lymphoma kinase (ALK), c-ros oncogene 1 kinase (ROS1), and pan-tropomyosin receptor kinases (Pan-TRKs) inhibitor. J Med Chem 59(7):3392–3408
Barter ZE, Tucker GT, Rowland-Yeo KJCp, (2013) Differences in cytochrome p450-mediated pharmacokinetics between chinese and caucasian populations predicted by mechanistic physiologically based pharmacokinetic modelling. Clin Pharmacokinet 52: 1085-1100
Hughes JH, Upton RN, Reuter SE et al (2019) Development of a physiologically based pharmacokinetic model for intravenous lenalidomide in mice. Cancer Chemother Pharmacol 84:1073–1087
Gao D, Wang G, Wu H et al (2023) Prediction for Plasma Trough Concentration and Optimal Dosing of Imatinib under Multiple Clinical Situations Using Physiologically Based Pharmacokinetic Modeling. ACS Omega.
Drilon A, Siena S, Ou S-HI et al (2017) Safety and antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I Trials (ALKA-372-001 and STARTRK-1) Entrectinib in NTRK-, ROS1-, or ALK-rearranged cancers. Cancer Discov 7(4):400–409
Meneses-Lorente G, Bentley D, Guerini E et al (2021) Characterization of the pharmacokinetics of entrectinib and its active M5 metabolite in healthy volunteers and patients with solid tumors. Invest New Drugs 39:803–811
Meneses-Lorente G, Fowler S, Guerini E et al (2022) In vitro and clinical investigations to determine the drug-drug interaction potential of entrectinib, a small molecule inhibitor of neurotrophic tyrosine receptor kinase (NTRK). Invest New Drugs 40(1):68–80
Mayr L, Guntner AS, Madlener S et al (2020) Cerebrospinal fluid penetration and combination therapy of entrectinib for disseminated ROS1/NTRK fusion-positive pediatric high-grade glioma. J Pers Med 10(4):290
Mercier F, Djebli N, González-Sales M et al (2022) Efficacy and safety exposure–response analyses of entrectinib in patients with advanced or metastatic solid tumors. Cancer Chemother Pharmacol 89(3):363–372
Food and Drug Administration (FDA). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/210259Orig1s000MultidisciplineR.pdf (accessed on 1, May, 2023).
Salerno SN, Edginton A, Gerhart JG et al (2021) Physiologically-based pharmacokinetic modeling characterizes the CYP3A-mediated drug-drug interaction between fluconazole and sildenafil in infants. Clin Pharmacol Ther 109(1):253–262
McCormick A, Swaisland H, Reddy VP et al (2018) In vitro evaluation of the inhibition and induction potential of olaparib, a potent poly (ADP-ribose) polymerase inhibitor, on cytochrome P450. Xenobiotica 48(6):555–564
Kuramoto S, Kato M, Shindoh H et al (2017) Simple evaluation method for CYP3A4 induction from human hepatocytes: the relative factor approach with an induction detection limit concentration based on the Emax model. Drug Metab Dispos 45(11):1139–1145
Schwenger E, Reddy VP, Moorthy G et al (2018) Harnessing meta-analysis to refine an oncology patient population for physiology-based pharmacokinetic modeling of drugs. Clin Pharmacol Ther 103(2):271–280
Dixon MR, Haukoos JS, Udani SM et al (2003) Carcinoembryonic antigen and albumin predict survival in patients with advanced colon and rectal cancer. Arch Surg 138(9):962–966
de LangeDanhof ECMJCP (2002) Considerations in the use of cerebrospinal fluid pharmacokinetics to predict brain target concentrations in the clinical setting: implications of the barriers between blood and brain. Clin Pharmacokinet 41:691–703
Yu J, Wang Y, Ragueneau-Majlessi IJDM et al (2022) Pharmacokinetic drug-drug interactions with drugs approved by the US Food and Drug Administration in 2020: mechanistic understanding and clinical recommendations. Drug Metab Dispos 50(1):1–7
Sun C, Nierman P, Kendall EK et al (2020) Clinical and biological implications of target occupancy in CLL treated with the BTK inhibitor acalabrutinib. Blood 136(1):93–105
Acknowledgements
We are grateful to all people for the contributions to the manuscript.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Investigation, methodology. and writing—original draft, LC; software and validation. NY; writing—original draft preparation, HY; Conceptualization, SZ and KZ, all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Yao, N., Yang, H. et al. Prediction of ROS1 and TRKA/B/C occupancy in plasma and cerebrospinal fluid for entrectinib alone and in DDIs using physiologically based pharmacokinetic (PBPK) modeling approach. Cancer Chemother Pharmacol 93, 107–119 (2024). https://doi.org/10.1007/s00280-023-04598-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04598-5