Abstract
Purpose
In animal experiments paclitaxel oleate associated with a cholesterol-rich nanoemulsion concentrated in the neoplastic tissues and showed reduced toxicity and increased antitumor activity compared with paclitaxel-Cremophor EL. Here, a clinical study was performed in breast cancer patients to evaluate the tumoral uptake, pharmacokinetics and toxicity of paclitaxel associated to nanoemulsions.
Methods
Twenty-four hours before mastectomy [3H]-paclitaxel oleate associated with [14C]-cholesteryl oleate-nanoemulsion or [3H]-paclitaxel in Cremophor EL were injected into five patients for collection of blood samples and fragments of tumor and normal breast tissue. A pilot clinical study of paclitaxel-nanoemulsion administered at 3-week intervals was performed in four breast cancer patients with refractory advanced disease at 175 and 220 mg/m2 dose levels.
Results
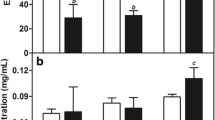
T1/2 of paclitaxel oleate associated to the nanoemulsion was greater than that of paclitaxel (t1/2 = 15.4 ± 4.7 and 3.5 ± 0.80 h). Uptake of the [14C]-cholesteryl ester nanoemulsion and [3H]-paclitaxel oleate by breast malignant tissue was threefold greater than the normal breast tissue and toxicity was minimal at the two dose levels.
Conclusions
Our results suggest that the paclitaxel-nanoemulsion preparation can be advantageous for use in the treatment of breast cancer because the pharmacokinetic parameters are improved, the drug is concentrated in the neoplastic tissue and the toxicity of paclitaxel is reduced.



Similar content being viewed by others
References
Ades A, Carvalho JP, Graziani SR, Amancio RF, Souen JS, Pinotti JÁ, Maranhão RC (2001) Uptake of a cholesterol-rich emulsion by neoplastic ovarian tissues. Gynecol Oncol 82(1):84–87
Crown J, O′Leary M (2000) The taxanes: an update. Lancet 355:1176–1178
Dias ML, Carvalho JP, Rodrigues DG, Graziani SR, Maranhão RC (2007) Pharmacokinetics and tumor uptake of a derivatized form of paclitaxel associated to a cholesterol-rich nanoemulsion (LDE) in patients with gynecologic cancers. Cancer Chemother Pharmacol 59:105–111
Dorr RT (1994) Pharmacology and toxicology of Cremophor EL diluent. Ann Pharmacother 28:S11–S14
Finley RS, Rowinsky EK (1994) Patient care issues: the management of paclitaxel-related toxicities. Ann Pharmacother 28:S27–S30
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gal D, MacDonald PC, Porter JC, Simpson ER (1981) Cholesterol metabolism in cancer cells in monolayer culture. Low density lipoprotein metabolism. Int J Cancer 28:315–319
Ginsburg GS, Small DM, Atkinson D (1982) Microemulsions of phospholipids and cholesterol esters. Protein-free models of low density lipoprotein. J Biol Chem 57:8216–8277
Goodman LS, Gilman A (2001) The pharmacological basis of therapeutics, 10th edn. McGraw-Hill, USA
Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy MJ (2005) Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23(31):7794–7803
Graziani SR, Igreja FAF, Hegg R, Meneghetti C, Brandizzi LI, Barboza R, Amâncio RF, Pinotti JA, Maranhão RC (2002) Uptake of a cholesterol-rich emulsion by breast cancer. Gynecol Oncol 85:493–497
Ho YK, Smith RG, Brown MS, Goldstein JL (1978) Low-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cells. Blood 52:1099–1114
Hungria VT, Latrilha MC, Rodrigues DG, Bydlowski SP, Chiattone CS, Maranhão RC (2004) Metabolism of a cholesterol-rich microemulsion (LDE) in patients with multiple myeloma and a preliminary clinical study of LDE as a drug vehicle for treatment of the disease. Cancer Chemother Pharmacol 53:51–60
Lo Prete AC, Maria DA, Rodrigues DG, Valduga CJ, Ibanez OC, Maranhão RC (2006) Evaluation in melanoma-bearing mice of an etoposide derivative associated to a cholesterol-rich nano-emulsion. J Pharm Pharmacol 58:801–808
Maranhão RC, Cesar TB, Pedroso MTB, Hirata MH, Mesquita CH (1993) Metabolic behavior in rats of a non protein microemulsion resembling LDL. Lipids 28:691–696
Maranhão RC, Garicochea B, Silva EL, Dorlhiac-Llacer P, Cadena SMS, Coelho IJC, Meneghetti JC, Pileggi FJC, Chamone DAF (1994) Plasma kinetics and biodistribution of a lipid emulsion resembling low-density lipoprotein in patients with acute leukemia. Cancer Res 54:4660–4666
Maranhão RC, Garicochea B, Silva EL, Llacer PD, Pileggi FJC, Chamone DAF (1992) Increased plasma removal of microemulsions resembling the lipid phase of low-density lipoproteins (LDL) in patients with acute myeloid leukemia: a possible new strategy for the treatment of the disease. Braz J Med Biol Res 25:1003–1007
Maranhão RC, Tavares ER, Padoveze AF, Valduga CJ, Oliveira TV, Rodrigues DG (2006) Paclitaxel associated with a lipid nanoemulsion (LDE) promotes atherosclerosis regression in the rabbit. Atheroscler Suppl 7:466
Maranhão RC, Graziani SR, Yamaguchi N, Melo RF, Latrilha MC, Rodrigues DG, Couto RD, Schreiber R, Buzaid AC (2002) Association of carmustine with a lipid emulsion: in vitro, in vivo and preliminary studies in cancer patients. Cancer Chemother Pharmacol 49:487–498
Matthews CME (1957) The theory of tracer experiments with 1331 I-labeled plasma proteins. Phys Med Biol 2:36–44
Mita AC, Olszanski AJ, Walovitch RC, Perez RP, MacKay K, Tuck DP, Simmons C, Hammond S, Mita MM, Beeram M, Stone AJ, Rowinsky EK, Lewis LD (2007) Phase I and pharmacokinetic study of AI-850, a novelmicroparticle hydrophobic drug delivery system for paclitaxel. Clin Cancer Res 13(11):3293–3301
Pinheiro KV, Hungria VT, Ficker ES, Valduga CJ, Mesquita CH, Maranhão RC (2006) Plasma kinetics of a cholesterol-rich microemulsion (LDE) in patients with Hodgkin’s and non-Hodgkin’s lymphoma and a preliminary study on the toxicity of etoposide associated with LDE. Cancer Chemother Pharmacol 57:624–630
Rodrigues DG, Covolan CC, Coradi ST, Barboza R, Maranhão RC (2002) Use of a cholesterol-rich emulsion that binds to low-density lipoprotein receptors as a vehicle for paclitaxel. J Pharm Pharmacol 54:765–772
Rodrigues DG, Maria DA, Fernandes DC, Valduga CJ, Couto RD, Ibañez OC, Maranhão RC (2005) Improvement of paclitaxel therapeutic index by derivatization and association to a cholesterol-rich microemulsion: in vitro and in vivo studies. Cancer Chemother Pharmacol 55:565–576
Rowinsky EK, Chaudhry V, Cornblath DR, Donehower RC (1993) Neurotoxicity of taxol. J Natl Cancer Inst Monogr 15:107–115
Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, Layloff T, Viswanathan CT, Cook CE, McDowall RD, Pittman KA, Spector S (1991) Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur J Drug Metab Pharmacokinet 16(4):249–255
Smith RE, Brown AM, Mamounas EP, Anderson SJ, Lembersky BC, Atkins JH, Shibata HR, Baez L, Defusco PA, Davila E, Tip** SJ, Bearden JD, Thirlwell MP (1999) Randomized trial of 3-hour versus 24-hour infusion of high-dose paclitaxel in patients with metastatic or locally advanced breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-26. J Clin Oncol 17:3403–3411
Sowby FS (1984) Radiation protection. In: Limits for intakes of radionuclides by workers. IRCP publication 30. Part I. Pergamon, Oxford
Valduga CJ, Fernandes DC, Lo Prete AC, Azevedo CHM, Rodrigues DG, Maranhão RC (2003) Use of a cholesterol-rich microemulsion that binds to low-density lipoprotein receptors as vehicle for etoposide. J Pharm Pharmacol 55:1615–1622
Vitols S, Gahrton G, Öst A, Peterson C (1984) Elevated low-density lipoprotein receptor activity in leukemic cells with monocytic differentiation. Blood 63:1186–1193
Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR, Van Echo DA, Von Hoff DD, Leyland-Jones B (1990) Hypersensitivity reactions from taxol. J Clin Oncol 8:1263–1268
Wiernik PH, Schwartz EL, Einzig A, Strauman JJ, Lipton RB, Dutcher JP (1987) Phase I trial of taxol given as a 24-hour infusion every 21 days: responses observed in metastatic melanoma. J Clin Oncol 5:1232–12329
Wilson WH, Berg SL, Bryant G, Wittes RE, Bates S, Fojo A, Steinberg SM, Goldspiel BR, Herdt J, O’Shaughnessy J (1994) Paclitaxel in doxorubicin-refractory or mitoxantrone-refractory breast cancer: a phase I/II trial of 96-hour infusion. J Clin Oncol 12:1621–1629
Acknowledgments
The authors grateful to Ms. Maria das Dores Pereira for the technical assistance. This study was supported by Fundação do Amparo à Pesquisa do Estado de São Paulo (FAPESP) and The Zerbini Foundation, both in São Paulo, Brazil. Dr. Maranhão is a 1A Research Awardee of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasilia, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pires, L.A., Hegg, R., Valduga, C.J. et al. Use of cholesterol-rich nanoparticles that bind to lipoprotein receptors as a vehicle to paclitaxel in the treatment of breast cancer: pharmacokinetics, tumor uptake and a pilot clinical study. Cancer Chemother Pharmacol 63, 281–287 (2009). https://doi.org/10.1007/s00280-008-0738-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0738-2