Abstract
For relapsed acute myeloid leukemia (AML) patients who received allogeneic hematopoietic stem cell transplantation, donor lymphocyte infusion (DLI) is an effective therapy. However, the cell source of DLI remains a topic of debate. In this study, we aimed to compare the efficacy and safety of G-CSF mobilized cells (G-DLI) with conventionally collected DLI (C-DLI). A total of 81 patients (50 C-DLI vs. 31 G-DLI) were assessed for clinical outcomes. There were no statistically significant differences in the baseline characteristics between the two groups including AML risk, donor types, interval from relapse to DLI, and infused CD3+ cell count. Although not statistically significant, complete remission (CR) and chimerism conversion rates were higher in G-DLI than in C-DLI: 51.6% vs. 28.0%, P = 0.057 and 42.3% vs. 28.2%, P = 0.363, respectively. There was no difference in acute graft-versus-host disease (GVHD) incidence and severity of acute GVHD between the two groups. The median overall survival (OS) of the G-DLI and C-DLI groups was 139 days and 106 days, respectively (P = 0.58). In conclusion, G-DLI appears to be a safe and an equally efficacious substitute for C-DLI, which is more readily available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (alloSCT) represents a curative treatment option for many acute myeloid leukemia (AML) patients. Unfortunately, relapse remains the main cause of treatment failure and impedes the survival of patients. The optimal treatment strategy for relapse-after-alloSCT setting remains to be determined; donor lymphocyte infusion (DLI) offers an attractive option. Based on the theoretical graft-versus-leukemia (GVL) effect mediated by donor T-cells, DLI was first applied to patients who experienced a relapse after alloSCT to treat chronic myeloid leukemia in 1990. With its success, the field of DLI flourished and many patients who had the choices of either only going through a second transplant or to succumbing to their underlying hematologic disease are being saved [1,2,3,4].
There are, however, some unresolved issues and areas for improvements. In general, DLI involves the re-collection of peripheral blood lymphocytes from the initial donor. The main hurdle for this conventional donor lymphocyte infusion (C-DLI) is the availability and safety of the donor, as unexpected adverse events can occur upon repeated cycles of collection. The development of graft-versus-host disease (GVHD) and aplasia also poses a problem [5, 6]. Such concerns have led to an interest in using the excess cryopreserved cells collected from the first alloSCT as the source of granulocyte-colony stimulating factor (G-CSF) mobilized DLI. G-CSF mobilized DLI (G-DLI) is especially appealing as previous studies have shown that G-CSF mobilized peripheral blood progenitor cell infusion leads to superior disease-free survival without increasing GVHD rates in relapsed patients [7, 8]. Recognizing the insufficiency of data on the efficacy and safety of G-DLI in comparison to C-DLI, particularly in Asian patients, we carried out this study to determine whether G-DLI can be a safe substitute for C-DLI.
Methods
Patients
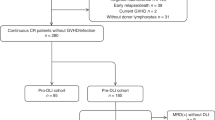
This is a retrospective longitudinal cohort study conducted between January 2001 and December 2017 at the Seoul National University Hospital. AML patients aged 16 years or older and undergoing therapeutic DLI for the first time due to a relapse after alloSCT were included in this study. Preemptive DLI, prophylactic DLI, and DLI for the treatment of engraftment failure were excluded. We included patients with morphologic relapse or extramedullary relapse in our analysis.
Their medical records were reviewed and analyzed for demographics, baseline disease characteristics, details of alloSCT, information and outcomes of DLI, and survival. The data on CD3+ cell count of the donor lymphocytes and CD3+/CD34+ cell count of the residual stem cells were also collected. This study was conducted according to the Declaration of Helsinki and was approved by the institutional review board of Seoul National University Hospital (IRB No. H-1903–020-1015). All authors had access to the study data and reviewed and approved this study.
Definitions
AML diagnosis was made according to the classification criteria used at the respective time of histological diagnosis [9]. The European LeukemiaNet classification was used for cytogenetic subgrou** [10], and since most patients were diagnosed before the next-generation sequencing era, only cytogenetic results by G-banding and fluorescence in situ hybridization (FISH) were considered for risk stratification. Treatment response and relapse were evaluated per the International Working Group guidelines [11]. Patients who survived for more than two years were defined as long-term survivors [12].
A chimerism study was performed by analyzing short tandem repeats (STRs) using PCR amplification [13]. Complete chimerism (CC) was defined as a complete changeover to donor cells. The coexistence of donor and recipient cells (1–99%) was defined as mixed chimerism (MC). When there were no donor cells found, the term “recipient cell only” was used. The chimerism conversion was defined as the change from MC or recipient cell only to CC after DLI.
Both newly developed acute GVHD after DLI and deterioration of known GVHD after DLI were documented. Acute GVHD grading was performed according to the Mount Sinai Acute GVHD International Consortium (MAGIC) standard criteria [14]. Fatal GVHD was defined as the death of a patient from uncontrolled GVHD. Chronic GVHD was classified as mild, moderate, or severe according to the 2014 National Institutes of Health consensus criteria [15].
DLI
In this study, C-DLI was defined as the infusion of lymphocytes additionally collected from an unstimulated donor who was the previous alloSCT donor, whereas G-DLI was defined as the infusion of remnant stem cells that were cryopreserved after the initial alloSCT without prophylactic immunosuppression conditioning [4]. Patients with remnant stem cells cryopreserved after previous alloSCT received G-DLI. The remaining peripheral blood stem cells from the initial alloSCT were cryopreserved − 190 °C, and these were the cell sources of G-DLI. Cryopreserved peripheral blood stem cells were thawed at 37 °C prior to infusion. The salvage chemotherapy for the relapsed patient was used per the attending physician’s decision. And patients with a history of immunosuppressant use before one month of relapse were included in the analysis.
Statistical analysis
Statistics differences between groups were assessed by using Student’s t test or one-way analysis of variance for continuous variables, and Pearson’s chi-square test for categorical variables. Survival analysis was performed using the Kaplan–Meier method. Overall survival (OS) was defined as the time from the first DLI to death from any cause. If patients survived without confirmed death or progression, survival was censored at the latest date of follow-up; data available up to July 2019 were used. Univariate and multivariate proportional hazards regression models were used to identify independent risk factors for OS by using Cox proportional hazard models. A stepwise backward procedure was used to construct a set of independent predictors at each endpoint. All predictors with P value below 0.10 were considered, and sequentially removed if the P value in the multiple model was above 0.05. All data were analyzed using R version 3.6.0 (The R foundation, Vienna, Austria). P values of < 0.05 were considered statistically significant.
Results
Patient characteristics
As shown in Table 1 and between the C-DLI and G-DLI groups, there were no significant differences in the baseline characteristics including AML risk, alloSCT setting, used conditioning regimen, and status at DLI. Most patients (93.8%) received the salvage chemotherapy before DLI, and the details of chemotherapy used are summarized in Supplemental Table 1. Chemotherapies commonly used for induction were used in relapsed patients, and among them, standard-dose cytarabine with idarubicin was the most used (43.2%). Of the 76 patients who received chemotherapy before DLI, 66 patients underwent DLI without evaluation after chemotherapy, and 10 patients were evaluated for disease status before DLI. Nine patients underwent bone marrow examination, of which 4 patients had persistent disease, 3 patients had inconclusive for remission because of low cellularity, and 2 patients obtained bone marrow remission. One patient was assessed with chimerism study and had complete chimerism. Of the patients who did not receive salvage chemotherapy, 4 received DLI immediately with early relapse, and one received DLI with extramedullary relapse.
Over 80% of the patients underwent alloSCT from full-matched donors and 72.9% of the patients were in complete remission (CR) at the time of alloSCT. Among the partially matched unrelated donors, there were three 9 of 10 HLA-matched donors in the C-DLI group, two 9 of 10 HLA-matched donors in the G-DLI group, and one 8 of 10 HLA-matched donor in the G-DLI group. Among the patients who underwent alloSCT from haploidentical donors, one patient of the C-DLI group received busulfan–cyclophosphamide–antithymocyte globulin conditioning, and the remaining 5 patients received busulfan–fludarabine–antithymocyte globulin conditioning. There were 2 patients who underwent alloSCT despite harboring favorable cytogenetic risk: one did not achieve CR after second induction and the other progressed during consolidation. At the time of DLI, the majority of the patients (82.7%) showed mixed chimerism, and 22.2% of the patients had active GVHD. Among the 6 patients with CC, 5 underwent DLI due to extramedullary relapse and 1 showed increased blast count (8%) on bone marrow examination. About 70% of patients were using ISA one month before relapse, but all discontinued after relapse was confirmed.
Outcomes of DLI
There was no difference in the number of cells infused as shown in Table 2. The median CD3+ cell count for C-DLI and G-DLI groups were 1.044 × 108/kg and 0.855 × 108/kg, respectively (P = 0.356). The patients with the maximum (6.9 × 108/kg) and minimum (0.1 × 108/kg) number of CD3+ cell counts were both in the C-DLI group. The median CD34+ cell count for the G-DLI group was 2.34 × 106/kg. There was no significant difference between the CD3+ cell counts used in 22 DLIs conducted before 2011 and 56 DLIs conducted thereafter. (0.76 × 108/kg and 1.06 × 108/kg, respectively, P = 0.181).
Among the 81 patients assessed, 30 (37%) achieved bone marrow remission after cell therapy. G-DLI showed a trend towards increased CR rate (51.6% vs. 28%); however, difference did not reach statistical significance (P = 0.057). Data on chimerism conversion after DLI were available from 65 patients (39 C-DLI and 26 G-DLI). A higher chimerism conversion rate was noted in the G-DLI group (11/26, 42.3%) than in C-DLI (11/39, 28.2%), but there was no statistically significant difference (P = 0.363). Among 3 patients who had recipient cells only chimerism at chimerism analyses, 2 patients died without chimerism study, and one patient got chimerism conversion after DLI. The one who got chimerism conversion survived over 300 days after DLI. One patient did not recover from bone marrow aplasia and died of pneumonia on day 13 from DLI. The other patient recovered from neutropenia 5 days after DLI, but blasts began to appear again in the peripheral blood a month later. He received two additional chemotherapy treatments and two more DLIs, but died from intracranial hemorrhage 180 days after the initial DLI.
There was no difference in the number of patients with persistent aplasia between the two groups(26.0%, 32.3%, respectively, P = 0.724). Among patients with persistent aplasia after DLI, the mean value of percentages of recipient cells in patients with mixed chimerism before DLI did not show significant difference between two groups (65.4% and 54.4%, respectively, P = 0.463). And, there was no difference in time from DLI to the first day of three consecutive days of peripheral blood neutrophil count of > 500 × 106/L (9 days, 8 days, respectively, P = 0.228).
The median overall survival after DLI was 3.5 months (range, 0.1–56.9 months) for the entire group (Table 2 and Fig. 1a). There were no differences in 1-year OS rates between the C-DLI and G-DLI groups: 20.0% and 22.6%, respectively (Fig. 1b). Patients who achieved bone marrow remission and/or chimerism conversion after DLI presented better survival than those who did not. More specifically, the median OS of patients who achieved bone marrow remission was 12 months compared to 2 months in those who did not (P < 0.001, Fig. 1c). As for chimerism conversion, patients who achieved CC after DLI showed significantly longer survival compared to those who did not (median 7.4 months vs. 2.1 months, respectively, P = 0.01) as shown in Fig. 1d. From multivariate analyses (Table 3), persistent disease after the first DLI (bone marrow remission vs. persistent, HR 4.85, 95% CI 2.40–9.80, P < 0.001), alloSCT donor type (matched sibling donor vs. haplo-identical donor, HR 2.68, 95% CI 1.10–6.48, P = 0.036), and shorter interval from transplantation to relapse (over 5 months vs. within 5 months, HR 1.68, 95% CI 1.03–2.74, P = 0.037) were considered as prognostic factors of OS. Whether DLI was performed once or additional treatment was not a good prognostic factor, which is because the response to treatment and the additional treatment were heterogenous. Among the patients who received additional treatment in the C-DLI group, 12 patients received additional chemotherapy and DLI, 5 patients received secondary transplantation. In the G-DLI group, 15 patients received additional chemotherapy and DLI, 2 patients received secondary transplantation.
GVHD
Among the patients, 35 (43.2%) showed signs of acute GVHD after DLI (Table 2), but there was no significant difference in the incidence rates between the C-DLI and G-DLI groups. Among them, 18 patients experienced aggravation of pre-existing GVHD. Of the 11 patients in the C-DLI group who had GVHD before DLI, 8 patients experienced aggravation of GVHD (72.7%). In the G-DLI group, 4 out of 7 patients (57.1%) experienced exacerbation of GVHD. There was a trend towards the increased grades III-IV acute GVHD in the C-DLI group (20%) compared with the G-DLI group (9.7%), but a similar incidence of fatal GVHD between the 2 groups (8.0% vs. 6.5%, respectively, P = 0.414). Although there were differences between patients, the main treatment for acute GVHD after DLI was the use of steroids and ISA.
Long-term survivors
There were 10 patients who were classified as long-term survivors (patients who survived longer than 2 years after DLI), among whom 6 and 4 underwent C-DLI and G-DLI, respectively. Table 4 shows details about these 10 patients. All 10 patients received salvage chemotherapy before DLI. There were 5 patients who remained alive at the time of data collection. There were 2 patients (patients 3 and 6) who had long remission duration but ultimately experienced central nervous system relapse and died. Three patients (patients 4, 8, and 9) underwent a second alloSCT from another donor after achieving bone marrow remission with DLI. Patient 4 and patient 8 maintained bone marrow remission and received a second alloSCT from a full matched unrelated donor. Patient 9 underwent second alloSCT due to CNS relapse 5 months after DLI. In the case of patient 7, the first bone marrow examination after DLI showed an slight increase in blast cells, which was suspected of being persistent disease. However, as a result of follow-up bone marrow examination without any additional treatment, bone marrow remission was determined.
Discussion
The importance of this study are that (1) as reported, we showed that DLI is a viable salvage option for AML patients relapsed after alloSCT, (2) G-CSF mobilized DLI yields a prognosis similar to that of conventional DLI, and (3) cryopreservation of graft content does not negatively affect the outcomes of DLI. In fact, G-DLI showed trends towards better CR rates and chimerism conversion rates compared to C-DLI without increasing GVHD incidence. Since G-DLI uses remnant stem cells collected from a previous alloSCT, there is no need for donor revalidation and additional collection, which also thereby provides time and economic benefits. Since this study did not consider the duration of salvage chemotherapy before DLI, it was not possible to see the difference in consumption time according to the two cell sources. Therefore, additional studies are needed to see if there is a difference in preparation time until DLI is administered between the two groups. Moreover, the G-DLI utilized in our study were cryopreserved and all the C-DLI were fresh. With no formal study on the impact of graft content cryopreservation on clinical outcomes, we hereby provide real-world evidence that it is safe to use cryopreserved products for later treatment.
G-CSF induces the mobilization of dendritic cells in humans and subsequently makes the dendritic cells available in peripheral lymphoid organs. This leads to the presentation of self-peptides and Th2 polarization in the donor [16, 17]. Since donor Th2 cells are less likely to induce aGVHD, the mobilization by G-CSF have been associated with reduced severity of aGVHD [16, 18, 19]. In line with such findings, patients who underwent G-DLI showed less grades III-IV aGVHD after DLI (Table 2). When dealing with patients relapsing after an alloSCT, attending physicians and patients alike may suffer from heavy emotional and physical burden of GVHD occurrence. In this regard, G-DLI may have certain advantages. Importantly, attenuated GVHD severity does not mean that G-CSF mobilized grafts lose their GVL effects. In fact, a different mechanism mainly concerning the perforin-dependent pathway [20] is responsible for GVL; thus, G-CSF mobilized grafts are able to retain their GVL effects while benefiting from decreased GVHD severity. Likewise, we observed similar, if not superior, CR or CC rates between G-DLI and C-DLI. In this study, patients treated with both kinds of DLI showed short duration of neutropenia compared to previous study that treating relapsed AML patients with chemotherapy followed by G-CSF primed DLI [7]. However, there was no significant difference between the C-DLI group and G-DLI group. This suggests that the presence of stem cells in the DLI product and the period of neutropenia may not be related. Further controlled studies are required.
As previously reported [21,22,23], DLI proved to be a promising salvage option with 1-year survival rates of over 20% (22.6% and 20.0% for the G-DLI and C-DLI groups, respectively). Several factors such as the achievement of hematological remission at DLI, favorable cytogenetics, and a longer duration of remission after alloSCT have been associated with good prognosis after DLI [24, 25]. Likewise, we recognized a longer interval from alloSCT to relapse and bone marrow remission after DLI as good prognostic factors (Table 3). Although alloSCT with haplo-identical donors was observed as a poor prognostic factor, careful interpretation is warranted. All of the alloSCT from haplo-donors was carried out prior to 2010; thus, these patients did not benefit from the advances in GVHD prophylaxis regimens including post-cyclophosphamide and improved infection control methodologies. Considering the fact that 4 of the 6 haplo-cases died due to GVHD and 2 due to infection within 60 days of DLI, this finding warrants further investigations. We wanted to ensure that patients received alloSCT and DLI from haplo-identical donors did not act as a bias. Therefore, baseline characteristics were analyzed between the two groups except for the six patients, and survival analysis was additionally performed. As a result, there was no significant difference from that of the entire patient group. The results are presented in supplemental Table 2 and supplemental Fig. 1.
In this study, 6 patients had CC at the baseline chimerism study. One of them had AML relapse while maintaining CC. The patient’s initial alloSCT donor was a matched sibling donor. It appears that some genetic abnormalites from donor cell can trigger the development of donor cell origin AML. After reinduction chemotherapy and the first DLI, the patient received chemotherapy and DLI once more and achieved bone marrow CR. However, cord compression occurred due to chloroma in the spine, and after surgery, the patient died of pneumonia. The remaining five patients with CC had extramedullary relapses. The treatment of extramedullary relapses has not yet been established. The DLI is considered as one of the treatment options [26, 27]. However, in sites where immune escape is possible, the GVL effect is known to be diminished, which would result in the less efficacy of the DLI in these sites [28].
One of the most obvious limitations of this study is its retrospective nature. The patients who will undergo C-DLI or G-DLI were not classified according to the certain criteria, but were analyzed retrospectively. Furthermore, although 81 cases of DLI are not trivial for a single center, it may perhaps be a little small to draw statistically powerful conclusions. Additionally, for G-DLI, the cryopreserved CD34+ cell count was not based on thawing and remeasurement, but was estimated based on the amount collected during the initial alloSCT. As for the limitations in terms of the effectiveness of DLI, especially in patients who did not have bone marrow remission or complete chimerism after DLI, the median overall survival was very short, about 2 months. As a result of this, in the case of patients who do not obtain remission with DLI, additional treatment should be considered as soon as possible. On the other hand, most of the patients in this study received salvage chemotherapy before DLI. In most cases, DLI is rapidly followed after chemotherapy, so hematological remission status cannot be assessed in the meantime. Lastly, since the duration of the study period spans from 2001 to 2017, many aspects of acute leukemia diagnosis and treatment, including risk stratification, use of target therapies, and supportive care, have altered over the course of time. However, we believe these shortcomings do not diminish the importance of our findings that can be readily incorporated into clinical practice.
In conclusion, we provide evidence that G-DLI is a readily available option that is worth considering for AML patients who have had a relapse after alloSCT. Further investigations on optimizing DLI use should ensue, but in the absence of established guidelines, this study provides physicians with better insights into clinical experience with DLI use.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
This is not applicable.
References
Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, van Rhee F, Mittermueller J, de Witte T, Holler E, Ansari H, European Group for B, Marrow Transplantation Working Party Chronic L (1995) Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 86(5):2041–2050
Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, Drobyski WR, Barrett AJ, Porter DL, Giralt S, Leis J, Holmes HE, Johnson M, Horowitz M, Collins RH Jr (2002) Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol 20(2):405–412. https://doi.org/10.1200/JCO.2002.20.2.405
Michallet AS, Nicolini F, Furst S, Le QH, Dubois V, Hayette S, Bourgeot JP, Tremisi JP, Thomas X, Gebuhrer L, Michallet M (2005) Outcome and long-term follow-up of alloreactive donor lymphocyte infusions given for relapse after myeloablative allogeneic hematopoietic stem cell transplantations (HSCT). Bone Marrow Transplant 35(6):601–608. https://doi.org/10.1038/sj.bmt.1704807
Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, Volin L, Gurman G, Maertens J, Bordigoni P, Holler E, Ehninger G, Polge E, Gorin NC, Kolb HJ, Rocha V, Party EALW (2007) Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol 25(31):4938–4945. https://doi.org/10.1200/JCO.2007.11.6053
Deol A, Lum LG (2010) Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev 36(7):528–538. https://doi.org/10.1016/j.ctrv.2010.03.004
Chang YJ, Huang XJ (2013) Donor lymphocyte infusions for relapse after allogeneic transplantation: when, if and for whom? Blood Rev 27(1):55–62. https://doi.org/10.1016/j.blre.2012.11.002
Choi SJ, Lee JH, Lee JH, Kim S, Seol M, Lee YS, Lee JS, Kim WK, Chi HS, Lee KH (2004) Treatment of relapsed acute myeloid leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a high incidence of isolated extramedullary relapse. Leukemia 18(11):1789–1797. https://doi.org/10.1038/sj.leu.2403523
Hasskarl J, Zerweck A, Wasch R, Ihorst G, Bertz H, Finke J (2012) Induction of graft versus malignancy effect after unrelated allogeneic PBSCT using donor lymphocyte infusions derived from frozen aliquots of the original graft. Bone Marrow Transplant 47(2):277–282. https://doi.org/10.1038/bmt.2011.45
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114(5):937–951. https://doi.org/10.1182/blood-2009-03-209262
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447. https://doi.org/10.1182/blood-2016-08-733196
Creutzig U, Kaspers GJ (2004) Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 22(16):3432–3433. https://doi.org/10.1200/JCO.2004.99.116
Frassoni F, Labopin M, Gluckman E, Prentice HG, Gahrton G, Mandelli F, Carella M, Herve P, Gratwohl A, Goldman J et al (1994) Are patients with acute leukaemia, alive and well 2 years post bone marrow transplantation cured? A European survey. Acute Leukaemia Working Party of the European Group for Bone Marrow Transplantation (EBMT). Leukemia 8(6):924–928
Kristt D, Israeli M, Narinski R, Or H, Yaniv I, Stein J, Klein T (2005) Hematopoietic chimerism monitoring based on STRs: quantitative platform performance on sequential samples. J Biomol Tech 16(4):380–391
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, Chanswangphuwana C, Efebera YA, Holler E, Litzow M, Ordemann R, Qayed M, Renteria AS, Reshef R, Wolfl M, Chen YB, Goldstein S, Jagasia M, Locatelli F, Mielke S, Porter D, Schechter T, Shekhovtsova Z, Ferrara JL, Levine JE (2016) International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant 22(1):4–10. https://doi.org/10.1016/j.bbmt.2015.09.001
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME (2015) National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21(3):389-401 e381. https://doi.org/10.1016/j.bbmt.2014.12.001
Pan L, Delmonte J Jr, Jalonen CK, Ferrara JL (1995) Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood 86(12):4422–4429
Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C (2000) Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood 95(8):2484–2490
Krenger W, Snyder KM, Byon JC, Falzarano G, Ferrara JL (1995) Polarized type 2 alloreactive CD4+ and CD8+ donor T cells fail to induce experimental acute graft-versus-host disease. J Immunol 155(2):585–593
Zeng D, Dejbakhsh-Jones S, Strober S (1997) Granulocyte colony-stimulating factor reduces the capacity of blood mononuclear cells to induce graft-versus-host disease: impact on blood progenitor cell transplantation. Blood 90(1):453–463
Pan L, Teshima T, Hill GR, Bungard D, Brinson YS, Reddy VS, Cooke KR, Ferrara JL (1999) Granulocyte colony-stimulating factor-mobilized allogeneic stem cell transplantation maintains graft-versus-leukemia effects through a perforin-dependent pathway while preventing graft-versus-host disease. Blood 93(12):4071–4078
Zeidan AM, Forde PM, Symons H, Chen A, Smith BD, Pratz K, Carraway H, Gladstone DE, Fuchs EJ, Luznik L, Jones RJ, Bolanos-Meade J (2014) HLA-haploidentical donor lymphocyte infusions for patients with relapsed hematologic malignancies after related HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant 20(3):314–318. https://doi.org/10.1016/j.bbmt.2013.11.020
Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, Bunjes DW, Zhang MJ (2015) Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant 21(3):454–459. https://doi.org/10.1016/j.bbmt.2014.11.007
Ghiso A, Raiola AM, Gualandi F, Dominietto A, Varaldo R, Van Lint MT, Bregante S, Di Grazia C, Lamparelli T, Galaverna F, Stasia A, Luchetti S, Geroldi S, Grasso R, Colombo N, Bacigalupo A (2015) DLI after haploidentical BMT with post-transplant CY. Bone Marrow Transplant 50(1):56–61. https://doi.org/10.1038/bmt.2014.217
Huff CA, Fuchs EJ, Smith BD, Blackford A, Garrett-Mayer E, Brodsky RA, Flinn IW, Ambinder RF, Borrello IM, Matsui WH, Vogelsang GB, Griffin CA, Luznik L, Jones RJ (2006) Graft-versus-host reactions and the effectiveness of donor lymphocyte infusions. Biol Blood Marrow Transplant 12(4):414–421. https://doi.org/10.1016/j.bbmt.2005.11.520
Takami A, Yano S, Yokoyama H, Kuwatsuka Y, Yamaguchi T, Kanda Y, Morishima Y, Fukuda T, Miyazaki Y, Nakamae H, Tanaka J, Atsuta Y, Kanamori H (2014) Donor lymphocyte infusion for the treatment of relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: a retrospective analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 20(11):1785–1790. https://doi.org/10.1016/j.bbmt.2014.07.010
Kottaridis PD, Ketley N, Peggs K, Chakraverty R, Ralleigh G, Shaw P, Pezzella F, Goldstone AH, Devereux S, Mackinnon S (1999) An unusual case of intrapulmonary granulocytic sarcoma presenting as interstitial pneumonitis following allogeneic bone marrow transplantation for acute myeloid leukaemia and responding to donor lymphocyte infusion. Bone Marrow Transplant 24(7):807–809. https://doi.org/10.1038/sj.bmt.1701974
Harris AC, Kitko CL, Couriel DR, Braun TM, Choi SW, Magenau J, Mineishi S, Pawarode A, Yanik G, Levine JE (2013) Extramedullary relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Haematologica 98(2):179–184. https://doi.org/10.3324/haematol.2012.073189
Kolb HJ, Schmid C, Barrett AJ, Schendel DJ (2004) Graft-versus-leukemia reactions in allogeneic chimeras. Blood 103(3):767–776. https://doi.org/10.1182/blood-2003-02-0342
Author information
Authors and Affiliations
Contributions
W.C.P and J.M.B contributed equally to this work. They collected data, analyzed results, and wrote the paper. J.S.H, I.H.K, D.Y.S, and S.Y.P provided patient information and helped write the paper. Y.I.K and S.S.Y are co-correspondence authors for this work. Y.I.K and S.S.Y designed the research and wrote the paper.
Corresponding authors
Ethics declarations
Ethics approval
This study was conducted according to the Declaration of Helsinki, and was approved by the institutional review board of Seoul National University Hospital (IRB No. H-1903–020-1015).
Consent to participate
This study used only the medical records of patients. In addition, it was managed to prevent any patient's personal information from being exposed. Based on this, the study was permitted to proceed without patient consent at the IRB.
Conflicts of interest
The authors declare no competing financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presented in abstract form at the AACR Annual meeting 2018, Chicago, Illinois, 15 April 2018. This work is based on the master's thesis of Woochan Park.
Supplementary Information

Supplemental Fig. 1
Survival outcomes of 75 patients subjected to donor lymphocyte infusion (DLI) except haploidentical donors. (a) All patients, The median overall survival was 3.8 months (range, 0.1–56.9 months). (b) Overall survival (OS) according to DLI source. The median survival between 2 groups showed no significant differences (3.5 months vs. 4.5 months, P = 0.78). (c) OS according to bone marrow remission achievement after DLI; the median OS of patients who achieved bone marrow remission was 12.6 months compared to 2.05 months in those who did not (P < 0.001). (d) OS according to chimerism conversion status after DLI. The patients who achieved CC after DLI showed significantly longer survival compared to those who did not (median 7.7 months vs. 2.3 months, respectively, P = 0.014) (PNG 129 kb)
ESM 1
(DOCX 21 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, W., Byun, J.M., Hong, J. et al. Comparison of the effect of DLI according to cell sources in relapsed AML after allogeneic stem cell transplantation. Ann Hematol 102, 629–639 (2023). https://doi.org/10.1007/s00277-023-05093-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05093-w