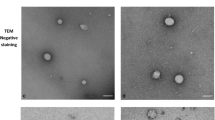
Abstract
The early death, which is more common in acute promyelocytic leukemia (APL) patients rather than other types of acute myelocytic leukemia (AML) highlights the importance of appropriate diagnostic method for early detection of this disease. The low sensitivity of the conventional methods, low tumor burden in some patients, and the need for bone marrow sampling are some of the diagnostic challenges on the way of proper detection of APL. Given these, we aimed to compare the efficacy of extracellular vesicles (EVs), as a diagnostic tool, with the existing methods. RT-PCR, qPCR, and flow cytometry were applied on EVs and their corresponding associated cellular component collected from 18 APL new cases, 23 patients with minimal residual disease (MRD), and NB4 cell line. RT-PCR results were positive in both cellular and vesicular components of all new cases, NB4 cells, and EVs in contrary to MRD cases. Normalized copy numbers (NCN) of PML-RARα were 5100 and 3950 for cell and EVs, respectively (p < 0.05). There was a significant difference in the NCN of PML-RARα between cells and EVs in BM samples. Investigating the effect of storage at room temperature revealed that PML-RARα level was retained near to the baseline level in EVs, but there was a significant reduction in its copy number in the cellular component during 7 days. Taken together, given to the acceptable stability, EVs could be introduced as a non-invasive liquid biopsy that alongside existing methods could remarkably change the paradigm of APL diagnostic approaches.









Similar content being viewed by others
References
Bally C, Fadlallah J, Leverger G, Bertrand Y, Robert A, Baruchel A, Guerci A, Recher C, Raffoux E, Thomas X (2012) Outcome of acute promyelocytic leukemia (APL) in children and adolescents: an analysis in two consecutive trials of the European APL Group. J Clin Oncol 30(14):1641–1646
Rodriguez-Rodriguez S, Demichelis-Gómez R, Diaz-Huizar MJ, Guerrero-Torres L, Pomerantz A, del Pilar Ortiz-Vilchis M, Aguayo A (2017) Cost-effectiveness of the regimen proposed by the international consortium on acute promyelocyticleukemia in the treatment of newly diagnosed patients with acute promyelocytic leukemia. Am Soc Hematology
Fenaux P, Tallman MS, Estey EH, Löwenberg B, Naoe T, Lengfelder E, Döhner H, Burnett AK, Chen SJ, Mathews V (2019) Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet
Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, Diverio D, Jones K, Aslett H, Batson E (2009) Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol 27(22):3650–3658
Hokland P, Ommen HB (2011) Towards individualized follow-up in adult acute myeloid leukemia in remission. Blood 117(9):2577–2584
Tobal K, Moore H, Macheta M, Yin JL (2001) Monitoring minimal residual disease and predicting relapse in APL by quantitating PML-RARα transcripts with a sensitive competitive RT-PCR method. Leukemia 15(7):1060
Wintrobe MM (2008) Wintrobe’s clinical hematology, vol 1. Lippincott Williams & Wilkins
Marrugo-Ramírez J, Mir M, Samitier J (2018) Blood-based cancer biomarkers in liquid biopsy: a promising non-invasive alternative to tissue biopsy. Int J Mol Sci 19(10):2877
Sasso L, Hosamuddin H, Emanueli C (2017) Extracellular vesicles at the cross-line between basic science and clinical needs. Microcirculation 24(1):e12333
van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19(4):213
Zhang W, **a W, Lv Z, **n Y, Ni C, Yang L (2017) Liquid biopsy for cancer: circulating tumor cells, circulating free DNA or exosomes? Cell Physiol Biochem 41(2):755–768
Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A (2014) Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 289(7):3869–3875
Caivano A, Laurenzana I, De Luca L, La Rocca F, Simeon V, Trino S, D’Auria F, Traficante A, Maietti M, Izzo T (2015) High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumor Biology 36(12):9739–9752
Yang C, Yang H, Liu J, Zhu L, Yu S, Zhang X (1815) Gao L (2019) Focus on exosomes: novel pathogenic components of leukemia. American journal of cancer research 9(8):1815
Kunz F, Kontopoulou E, Reinhardt K, Soldierer M, Strachan S, Reinhardt D, Thakur BK (2019) Detection of AML-specific mutations in pediatric patient plasma using extracellular vesicle–derived RNA. Ann Hematol 98(3):595–603
Kontopoulou E, Strachan S, Reinhardt K, Kunz F, Walter C, Walkenfort B, Jastrow H, Hasenberg M, Giebel B, von Neuhoff N (2020) Evaluation of dsDNA from extracellular vesicles (EVs) in pediatric AML diagnostics. Ann Hematol 1–17
Cai J, Han Y, Ren H, Chen C, He D, Zhou L, Eisner GM, Asico LD, Jose PA, Zeng C (2013) Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol 5(4):227–238
Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M (2011) Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-β1. Haematologica 96(9):1302–1309
Boyiadzis M, Whiteside TL (2015) Information transfer by exosomes: a new frontier in hematologic malignancies. Blood Rev 29(5):281–290
Sharifi H, Shafiee A, Molavi G, Razi E, Mousavi N, Sarvizadeh M, Taghizadeh M (2019) Leukemia-derived exosomes: bringing oncogenic signals to blood cells. J Cell Biochem 120(10):16307–16315
Li Y, Zhao J, Yu S, Wang Z, He X, Su Y, Guo T, Sheng H, Chen J, Zheng Q (2019) Extracellular vesicles long RNA sequencing reveals abundant mRNA, circRNA, and lncRNA in Human blood as potential biomarkers for cancer diagnosis. Clin Chem 65(6):798–808
Peng M, **e Y, Li X, Qian Y, Tu X, Yao X, Cheng F, Xu F, Kong D, He B (2019) Resectable lung lesions malignancy assessment and cancer detection by ultra-deep sequencing of targeted gene mutations in plasma cell-free DNA. J Med Genet 56(10):647–653
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750
Pospichalova V, Svoboda J, Dave Z, Kotrbova A, Kaiser K, Klemova D, Ilkovics L, Hampl A, Crha I, Jandakova E (2015) Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J Extracell Vesicles 4(1):25530
Van Dongen J, Macintyre E, Gabert J, Delabesse E, Rossi V, Saglio G, Gottardi E, Rambaldi A, Dotti G (1901) Griesinger F (1999) Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Leukemia 13(12):1901
Cassinat B, Zassadowski F, Balitrand N, Barbey C, Rain J, Fenaux P, Degos L, Vidaud M, Chomienne C (2000) Quantitation of minimal residual disease in acute promyelocytic leukemia patients with t (15; 17) translocation using real-time RT-PCR. Leukemia 14(2):324
Ghaffari S, Rostami S, Bashash D, Alimoghaddam K, Ghavamzadeh A (2006) Real-time PCR analysis of PML-RARα in newly diagnosed acute promyelocytic leukaemia patients treated with arsenic trioxide as a front-line therapy. Ann Oncol 17(10):1553–1559
Santamaría C, Chillón MC, Fernández C, Martín-Jiménez P, Balanzategui A, Sanz RG, San Miguel JF, González M-G (2007) Using quantification of the PML-RARα transcript to stratify the risk of relapse in patients with acute promyelocytic leukemia. Haematologica 92(3):315–322
Beillard E, Pallisgaard N, Van der Velden V, Bi W, Dee R, van der Schoot E, Delabesse E, Macintyre E, Gottardi E, Saglio G (2003) Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)–a Europe against cancer program. Leukemia 17(12):2474
Biccler JL, Østgård LSG, Severinsen MT, Marcher CW, Møller P, Schöllkopf C, Friis LS, Bøgsted M, Jakobsen LH, El-Galaly TC (2018) Evolution of relative survival for acute promyelocytic leukemia patients alive at landmark time-points: a population-based study. Leukemia:1
Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E, Faderl S, O’Brien S, Wierda W, Pierce S (2017) Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood 129(10):1275–1283
Hong C-S, Muller L, Whiteside TL, Boyiadzis M (2014) Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Front Immunol 5:160
Hong CS, Muller L, Boyiadzis M, Whiteside TL (2014) Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PloS one 9(8):e103310
Kucharzewska P, Belting M (2013) Emerging roles of extracellular vesicles in the adaptive response of tumour cells to microenvironmental stress. Journal of Extracellular Vesicles 2(1):20304
Lv L-H, Wan Y-L, Lin Y, Zhang W, Yang M, Li G-L, Lin H-M, Shang C-Z, Chen Y-J, Min J (2012) Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 287(19):15874–15885
Fang Y, Garnier D, Lee TH, D’Asti E, Montermini L, Meehan B, Rak J (2016) PML–RARa modulates the vascular signature of extracellular vesicles released by acute promyelocytic leukemia cells. Angiogenesis 19(1):25–38
Kang K-W, Jung J-H, Hur W, Park J, Shin H, Choi B, Jeong H, Kim DS, Yu ES, Lee SR (2018) The Potential of exosomes derived from chronic myelogenous leukaemia cells as a biomarker. Anticancer Res 38(7):3935–3942
Schuurhuis G, Heuser M, Freeman S, Béné M, Buccisano F, Cloos J, Grimwade D, Haferlach T, Hills R, Hourigan C (2018) Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party
Milani G, Lana T, Bresolin S, Aveic S, Pastò A, Frasson C, Te Kronnie G (2017) Expression profiling of circulating microvesicles reveals intercellular transmission of oncogenic pathways. Mol Cancer Res 15(6):683–695
Ghavamzadeh A, Alimoghaddam K, Rostami S, Ghaffari SH, Jahani M, Iravani M, Mousavi SA, Bahar B, Jalili M (2011) Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J Clin Oncol 29(20):2753–2757
Grimwade D, Coco FL (2002) Acute promyelocytic leukemia: a model for the role of molecular diagnosis and residual disease monitoring in directing treatment approach in acute myeloid leukemia. Leukemia 16(10):1959–1973
Boddu P, Jorgensen J, Kantarjian H, Borthakur G, Kadia T, Daver N, Alvarado Y, Pemmaraju N, Bose P, Naqvi K (2018) Achievement of a negative minimal residual disease state after hypomethylating agent therapy in older patients with AML reduces the risk of relapse. Leukemia 32(1):241–244
van der Velden VH, Boeckx N, Gonzalez M, Malec M, Barbany G, Lion T, Gottardi E, Pallisgaard N, Beillard E, Hop W (2004) Differential stability of control gene and fusion gene transcripts over time may hamper accurate quantification of minimal residual disease–a study within the Europe Against Cancer Program. Leukemia 18(4):884–886
Opitz L, Salinas-Riester G, Grade M, Jung K, Jo P, Emons G, Ghadimi BM, Beißbarth T, Gaedcke J (2010) Impact of RNA degradation on gene expression profiling. BMC Med Genomics 3(1):36
Prezeau N, Silvy M, Gabert J, Picard C (2006) Assessment of a new RNA stabilizing reagent (tempus blood RNA) for minimal residual disease in onco-hematology using the EAC protocol. Leuk Res 30(5):569–574
Ge Q, Zhou Y, Lu J, Bai Y, **e X, Lu Z (2014) miRNA in plasma exosome is stable under different storage conditions. Molecules 19(2):1568–1575
Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD, Mathivanan S (2013) Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 13(22):3354–3364
Acknowledgements
The authors would like to thank their dear colleagues from Hematopoietic Stem Cell and Bone Marrow Transplantation Research Center (HSCT), Shahid Beheshti University of Medical Sciences, Mr. Amin mirzaeian, Miss Bentolhoda Kohestani, and Miss Shaghayegh Shahsavan for their cooperation in data gathering. This article has been extracted from the thesis written by Mr. Mohieddin Barzegar in School of Allied Medical Science, Shahid Beheshti University of Medical Sciences (Registration No: 15919).
Funding
Funding was done by Shahid Beheshti University of Medical Science Registration No: 15919.
Author information
Authors and Affiliations
Contributions
Barzegar carried out the experiments, collected the samples, did the statistical analysis, and wrote the first version of the manuscript; Parkhihdeh, Rafiee, and Amiri drafted the manuscript and analyzed the data; Mohammadi, Allahbakhshian Farsani, and Rad conceived of the study, designed and coordinated it, and finalized the manuscript. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Ethical approval
The study was approved ethically by committee of ethics in by Shahid Beheshti University of Medical Science.
Informed consent
We have signed consent from participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 692 KB)
Rights and permissions
About this article
Cite this article
Barzegar, M., Farsani, M.A., Rafiee, M. et al. Acute promyelocytic leukemia derived extracellular vesicles conserve PML-RARα transcript from storage-inflicted degradation: a stable diagnosis tool in APL patients. Ann Hematol 100, 2241–2252 (2021). https://doi.org/10.1007/s00277-021-04579-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04579-9