Abstract
Purpose
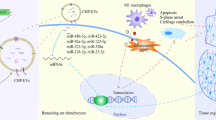
Adipose-derived mesenchymal stem cell (ADSC)-based therapies have been utilized for cartilage regeneration because of their multi-lineage differentiation ability. However, commonly used cartilage inducers such as the transforming growth factor beta-3 (TGF-β3) may be prone to cartilage dedifferentiation and hypertrophy. The directional differentiation of elastic cartilage is limited nowadays. Extracellular vesicles (EVs) have been reported to influence the specific differentiation of mesenchymal stem cells (MSCs) by reflecting the composition of the parental cells. However, the role of auricular chondrogenic-derived EVs (AC-EVs) in elastic chondrogenic differentiation of ADSCs has not yet been reported.
Results
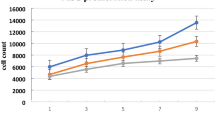
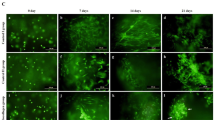
AC-EVs isolated from the external ears of swine exhibited a positive effect on cell proliferation and migration. Furthermore, AC-EVs efficiently promoted chondrogenic differentiation of ADSCs in pellet culture, as shown by the elevated levels of COL2A1, ACAN, and SOX-9 expression. Moreover, there was a significantly higher expression of elastin and a lower expression of the fibrotic marker COL1A1 in comparison with that achieved with TGF-β3. The staining results demonstrated that AC-EVs promoted the deposition of cartilage-specific matrix, which is in good concordance with the real-time polymerase chain reaction (RT-PCR) results.
Conclusions
Auricular chondrogenic-derived EVs are a crucial component in elastic chondrogenic differentiation and other biological behaviors of ADSCs, which may be a useful ingredient for cartilage tissue engineering and external ear reconstruction.
No Level Assigned
This journal requires that authors 42 assign a level of evidence to each submission to which 43 Evidence-Based Medicine rankings are applicable. This 44 excludes Review Articles, Book Reviews, and manuscripts 45 that concern Basic Science, Animal Studies, Cadaver 46 Studies, and Experimental Studies. For a full description of 47 these Evidence-Based Medicine ratings, please refer to the 48 Table oôf Contents or the online Instructions to Authors 49 www.springer.com/00266.




Similar content being viewed by others
Availability of Data and Materials
Not applicable
Code Availability
Not applicable
References
Kalluri R, Lebleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367(6478):eaau6977
Thery C, Witwer KW, Aikawa E et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750
Narayanan K, Kumar S, Padmanabhan P et al (2018) Lineage-specific exosomes could override extracellular matrix mediated human mesenchymal stem cell differentiation. Biomaterials 182:312–322
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8(4):315–317
Leblanc P, Arellano-Anaya ZE, Bernard E et al (2017) Isolation of exosomes and microvesicles from cell culture systems to study prion transmission. Methods Mol Biol 1545:153–176
Théry C, Witwer KW, Aikawa E et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750
Campo AA, Victor-Baldin A, Contreras-Mérida SM (2018) Surgical-based classification for microtia. J Craniofac Surg 29(6):1651–1654
Nuyen BA, Kandathil CK, Saltychev M et al (2021) The social perception of Microtia and auricular reconstruction. Laryngoscope 131(1):195–200
Wu G, Lu L, Ci Z et al (2022) Three-dimensional cartilage regeneration using engineered cartilage gel with a 3D-printed polycaprolactone framework. Front Bioeng Biotechnol 10:871508
Bružauskaitė I, Bironaitė D, Bagdonas E et al (2016) Scaffolds and cells for tissue regeneration: different scaffold pore sizes-different cell effects. Cytotechnology 68(3):355–369
Sterodimas A, de Faria J, Correa WE et al (2009) Tissue engineering and auricular reconstruction: a review. J Plast Reconstr Aesthet Surg 62(4):447–452
Song H, Zhao J, Cheng J et al (2021) Extracellular vesicles in chondrogenesis and cartilage regeneration. J Cell Mol Med 25(11):4883–4892
Woo CH, Kim HK, Jung GY et al (2020) Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles 9(1):1735249
Hu H, Dong L, Bu Z et al (2020) miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. J Extracell Vesicles 9(1):1778883
Ma K, Zhu B, Wang Z et al (2020) Articular chondrocyte-derived extracellular vesicles promote cartilage differentiation of human umbilical cord mesenchymal stem cells by activation of autophagy. J Nanobiotechnol 18(1):163
Chen Y, Xue K, Zhang X et al (2018) Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res Ther 9(1):318
Li Z, Wang Y, **ang S et al (2020) Chondrocytes-derived exosomal miR-8485 regulated the Wnt/β-catenin pathways to promote chondrogenic differentiation of BMSCs. Biochem Biophys Res Commun 523(2):506–513
**e Y, Liu X, Wang S et al (2019) Proper mechanical stimulation improve the chondrogenic differentiation of mesenchymal stem cells: Improve the viscoelasticity and chondrogenic phenotype. Biomed Pharmacother 115:108935
Schätti O, Grad S, Goldhahn J et al (2011) A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur Cell Mater 22:214–225
Kurenkova AD, Romanova IA, Kibirskiy P et al (2022) Strategies to convert cells into hyaline cartilage: magic spells for adult stem cells. Int J Mol Sci 23(19):11169
K F, E M, Pg R, et al (2021) Characterisation of ovine bone marrow-derived stromal cells (oBMSC) and evaluation of Chondrogenically induced micro-pellets for cartilage tissue repair in vive. Stem Cell Res Ther 12(1):26
Deng Y, Lei G, Lin Z et al (2019) Engineering hyaline cartilage from mesenchymal stem cells with low hypertrophy potential via modulation of culture conditions and Wnt/β-catenin pathwa. Biomaterials 192:569–578
Pomerantseva I, Bichara DA, Tseng A et al (2016) Ear-shaped stable auricular cartilage engineered from extensively expanded chondrocytes in an immunocompetent experimental animal model. Tissue Eng Part A 22(3–4):197–207
Oba T, Okamoto S, Ueno Y et al (2022) In vitro elastic cartilage reconstruction using human auricular perichondrial chondroprogenitor cell-derived micro 3D spheroids. J Tissue Eng 13:204
Yanaga H, Imai K, Koga M et al (2012) Cell-engineered human elastic chondrocytes regenerate natural scaffold in vitro and neocartilage with neoperichondrium in the human body post-transplantation. Tissue Eng Part A 18(19–20):2020–2029
Tseng A, Pomerantseva I, Cronce MJ et al (2014) Extensively expanded auricular chondrocytes form neocartilage in vivo. Cartilage 5(4):241–251
Malivce E, Kregar N, Barlivc A et al (2009) Comparison of articular and auricular cartilage as a cell source for the autologous chondrocyte implantatio. J Orthopaed Res 27(7):943–948
Cc W, Ch C, Lh C et al (2018) Facilitating in vivo articular cartilage repair by tissue-engineered cartilage grafts produced from auricular chondrocytes. Am J Sports Med 46(3):713–727
Lohan A, Marzahn U, Sayed KE et al (2014) Osteochondral articular defect repair using auricle-derived autologous chondrocytes in a rabbit model. Ann Anatomy Anatom Anzeig 196(5):317–326
Shin H, Lee MN, Choung JS et al (2016) Focal adhesion assembly induces phenotypic changes and dedifferentiation in chondrocytes. J Cell Physio 231(8):1822–1831
Xu R, Greening DW, Zhu HJ et al (2016) Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest 126(4):1152–1162
Lai RC, Yeo RWY, Lim SK (2015) Mesenchymal stem cell exosome. Semin Cell Dev Biol 40:82–88
Colao IL, Corteling R, Bracewell D et al (2018) Manufacturing exosomes: a promising therapeutic platfor. Trends Mol Med 24(3):242–256
Mao G, Zhang Z, Hu S et al (2018) Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther 9(1):247
H S, S H, Z Z, et al (2019) Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J Cell Biochem 120(1):171–181
Wang R, Xu B, Xu H (2018) TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 17(24):2756–2765
Mao G, Hu S, Zhang Z et al (2018) Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J Cell Mol Med 22(11):5354–5366
Liu N, Wang W, Zhao Z et al (2014) Autophagy in human articular chondrocytes is cytoprotective following glucocorticoid stimulation. Mol Med Rep 9(6):2166–2172
Shen C, Cai GQ, Peng JP et al (2015) Autophagy protects chondrocytes from glucocorticoids-induced apoptosis via ROS/Akt/FOXO3 signaling. Osteoarthritis Cartil 23(12):2279–2287
JK, Lunney, Van Goor A, Kristen E, et al (2021) Importance of the pig as a human biomedical model. Science Translat Med 13(621):5758
Riazifar M, Pone EJ, Lötvall J et al (2017) Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol 57:125–154
Li J, Chen X, Yi J et al (2016) Identification and characterization of 293T cell-derived exosomes by profiling the protein, mRNA and MicroRNA components. PLoS ONE 11(9):e0163043
Chen TS, Arslan F, Yin Y et al (2011) Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med 9:47
Acknowledgement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Contributions
RG, JF
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Consent to Participate
Not applicable
Consent to Publication
Not applicable
Ethical Approval
All the procedures were approved by the Ethics Committee of the Plastic Surgery Hospital 2022[217]
Human and Animal Rights
All the procedures in this study were approved by the Ethics Committee of the Plastic Surgery Hospital. The animal experiments of the project complied with the principles of animal protection, animal welfare, and ethics, and it complied with the relevant regulations of the national laboratory animal welfare ethics. 2022[217].
Informed Consent
For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, R., Fan, J. Extracellular Vesicles Derived from Auricular Chondrocytes Facilitate Cartilage Differentiation of Adipose-Derived Mesenchymal Stem Cells. Aesth Plast Surg 47, 2823–2832 (2023). https://doi.org/10.1007/s00266-023-03292-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-023-03292-4