Abstract
Objectives
To determine the associated thromboembolism risk with adding immune checkpoint inhibitors (ICI) to platinum combination chemotherapy compared with platinum combination chemotherapy alone in patients with advanced non-small cell lung cancer.
Materials and methods
This study identified 75,807 patients with advanced non-small cell lung cancer from the Japanese Diagnosis Procedure Combination database who started platinum combination chemotherapy between July 2010 and March 2021. The incidence of venous thromboembolism (VTE), arterial thromboembolism (ATE), and all-cause mortality within 6 months after commencing platinum combination chemotherapy was compared between patients receiving chemotherapy with ICI (ICI group, n = 7,177) and without ICI (non-ICI group, n = 37,903). Survival time analysis was performed using the overlap weighting method with propensity scores to adjust for background factors. The subdistribution hazard ratio for develo** thromboembolism was calculated using the Fine-Gray model with death as a competing risk. The hazard ratio for all-cause mortality was also calculated using the Cox proportional hazards model.
Results
Overall, VTE and ATE occurred in 761 (1.0%) and 389 (0.51%) patients, respectively; mortality was 11.7%. Propensity score overlap weighting demonstrated that the subdistribution hazard ratio (95% confidence interval) for VTE and ATE in the ICI group was 1.27 (1.01–1.60) and 0.96 (0.67–1.36), respectively, compared with the non-ICI group. The mortality hazard ratio in the ICI group was 0.68 (0.62–0.74).
Conclusion
The addition of ICI to platinum combination therapy was associated with a higher risk of VTE compared with platinum combination therapy alone, while the risk of ATE might be comparable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer patients are at increased risk for venous thromboembolism (VTE) and arterial thromboembolism (ATE) [1, 2]. The risk of cancer-related thrombosis is multifactorial, and many risk factors have been reported [3]. Among them, anticancer agents such as platinum and angiogenesis inhibitors have been found to increase the risk of VTE and ATE [3,4,5].
There have been an increasing number of reports regarding the risk of thromboembolism with immune checkpoint inhibitors (ICIs) [6,7,8,9,10] because ICIs have revolutionized the treatment of malignancies (e.g., malignant melanoma, lung cancer, and renal cell carcinoma). ICIs could increase the levels of inflammatory cytokines [11] and enhance prothrombotic conditions by activating coagulation and impairing fibrinolysis [12,13,14]. A cohort study (n = 2,299) using data from a U.S. database demonstrated the cumulative 6-month VTE incidence following first-line treatment for non-small cell lung cancer in patients treated with ICIs alone, chemotherapy alone, and ICI plus chemotherapy was 8.1%, 10.9%, and 12.8%, respectively [15]. Regarding the risk of develo** ATE, a previous matched-pair cohort study (n = 5,684) reported a threefold higher risk of atherosclerotic cardiovascular events after initiation of ICI therapy [16]. However, none of these studies have examined the risk of thromboembolism with ICIs compared with conventional chemotherapy [17] despite the fact that additional administration of ICIs with platinum-based therapy for lung cancer is becoming common.
This study aimed to determine the risk of thromboembolism associated with adding ICIs to platinum combination chemotherapy compared with platinum combination chemotherapy alone in patients with advanced non-small cell lung cancer.
Materials and methods
Data source
This nationwide retrospective cohort study used the Japanese Diagnosis Procedure Combination database. The database includes discharge abstracts and administrative claims data for approximately 8,000,000 inpatient admissions from more than 1,200 hospitals throughout Japan. It covers about half of all patients admitted to acute care hospitals in Japan [18, 19]. All 82 academic hospitals are required to participate in the database, whereas the participation of community hospitals is voluntary.
The database contains the following information: unique hospital identifiers; patient age and sex; smoking history (including both current and former smoking status) at admission; body mass index (BMI) at admission; activities of daily living (ADL) at admission; dates of admission and discharge; length of hospital stay; in-hospital mortality; cancer stage; blood transfusions and medications; and interventional/surgical procedures indexed by original Japanese codes. Diagnoses, comorbidities, and complications are recorded using the International Classification of Diseases, Tenth Revision (ICD-10) codes, and Japanese text data. The database includes no laboratory data. A previous validation study showed good sensitivity and specificity for the diagnoses and procedures recorded in this database [18].
The need for informed consent for this study was waived because the patient database was anonymized. The study was approved by the Institutional Review Board of the University of Tokyo (Approval number: 3501– (5), May 19, 2021).
Patient selection
Patients hospitalized for the first administration of platinum combination therapy for advanced non-small cell lung cancer between July 1, 2010, and March 31, 2021, were identified. Non-small cell lung cancer was identified using the ICD-10 code of C34 and Japanese text data. Platinum combination therapy was defined as the following regimens, including cisplatin (CDDP) or carboplatin (CBDCA): (a) CDDP plus pemetrexed (PEM), (b) CBDCA plus PEM, (c) CBDCA plus nab-paclitaxel (nabPTX), (d) CBDCA plus paclitaxel (PTX), (e) CBDCA plus PTX plus bevacizumab (BEV), and (f) above-stated regimens plus ICI (pembrolizumab or atezolizumab). The ICIs used in each regimen were described.
Eligible patients were divided into the ICI and non-ICI groups. The ICI group included patients administered platinum combination regimens plus an ICI (pembrolizumab or atezolizumab), and the non-ICI group included those without ICI therapy. The following patients were excluded: (i) those with pulmonary sarcoma, pediatric pleuropulmonary blastoma, or pulmonary malignant melanoma; (ii) those aged less than 18 years; (iii) those who started chemotherapy after October 1, 2020 (because of the observation period being less than 6 months); (iv) those treated with multiple regimens in the same hospitalization; (v) those who received anticancer agents of the regimen on separate days; (vi) those who received multiple cycles of platinum combination regimens in the same hospitalization; (vii) those who were administered anticoagulants (direct oral anticoagulants [dabigatran, rivaroxaban, apixaban, and edoxaban] and warfarin) within the past 1 year; and (viii) those who had experienced VTE or ATE within the past 1 year.
Outcomes
The primary outcomes were VTE and ATE, requiring hospitalization within 6 months after the start of platinum combination chemotherapy. VTE included deep vein thrombosis (ICD-10 codes: I80.1, I80.2, I80.3, I80.8, I80.9, and I82.8) and pulmonary embolism (I26.0 and I26.9) [20]. According to previous studies, ATE was defined as ischemic heart disease (I20.0, I20.1, I20.8, I20.9, I21.0, I21.1, I21.2, I21.3, I21.4, and I21.9), ischemic brain disease (I63 and G45.9), and peripheral arterial occlusion (I74.0, I74.1, I74.2, I74.3, I74.4, I74.5, I74.8, and I74.9) [1, 8]. VTE or ATE onset was defined as the date when the patient received direct oral anticoagulants or warfarin for a VTE or ATE diagnosis. The secondary outcome was all-cause in-hospital death within 6 months after the start of platinum combination chemotherapy.
Covariates
Covariates were age, sex, BMI, weight, smoking status (nonsmoker, current/past smoker, missing), ADL at admission, combined small cell lung carcinoma, comorbidities, clinical cancer stage (III or IV), treatments before admission (molecular-targeted medications, dialysis, radiotherapy, and surgery within 6 months prior to the index hospitalization), pretreatment medications and procedures during the index hospitalization, baseline chemotherapy, days from admission to initiation of chemotherapy, type of hospital (academic hospital or non-academic hospital), and hospital volume.
Age was categorized into six groups: 18–39, 40–49, 50–59, 60–69, 70–79, and 80 or more years. BMI was categorized using the World Health Organization classifications: less than 18.5 kg/m2 (underweight), 18.5–24.9 kg/m2 (normal weight), 25.0–29.9 kg/m2 (overweight), and more than or equal to 30.0 kg/m2 (obese and severely obese). ADL at admission was assessed using the Barthel index and categorized into three groups: less than or equal to 40, 45–80, and 85–100 [21, 22]. Comorbidities were investigated using the ICD-10 codes (Supplemental Table 1) and assessed using the Charlson comorbidity index [23]. The index was categorized into three groups: less than or equal to 2, 3–4, and more than or equal to 5. Pretreatment medications included antihypertensives, antiplatelet drugs, antipsychotics, corticosteroids, estrogen preparations, non-steroidal anti-inflammatory drugs, proton pump inhibitors/potassium-competitive acid, and statins. Pretreatment procedures included central venous catheterization, radiotherapy, surgery, and transfusion of red cell concentrate. A previous study reported that CDDP-based regimens were associated with a higher risk of VTE than CBDCA-based regimens in patients with lung cancer [24]; therefore, to account for differences in the risk of thrombosis, we adjusted for the baseline chemotherapy regimens in the current analysis. Hospital volume was defined as the annual number of eligible patients at each hospital and categorized into tertiles with approximately equal numbers of patients in each group.
Statistical analysis
We conducted propensity-score overlap weighting to control for potential confounding factors. The overlap weighting analysis balanced the two groups by minimizing the asymptotic variance of the nonparametric estimates of the weighted average treatment effect within a class of weights [25,26,27,16], but the current study had a different population with BMIs less than 25 kg/m2in more than 80% of participants; this difference might have resulted in the low ATE incidence regardless of ICI administration in the current cohort. Additionally, the difference in chemotherapy combined with ICI in the present study may have affected ATE incidence. Platinum chemotherapy requires high-volume infusion and special attention to cardiac function [43]. Thus, clinicians would have selected patients with good cardiac function in the current population treated with platinum chemotherapy.
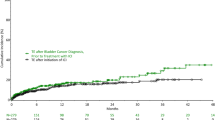
All-cause mortality within 6 months was significantly lower in the ICI group than in the non-ICI group (Fig. 2). The reduced mortality in the present study is similar to previous randomized controlled trials (e.g., the HR for mortality was 0.71 in KEYNOTE-407) [44,45,46,47]. These trials demonstrated long-term survival (approximately 17–22 months) in patients treated with ICI, whereas the current study revealed that ICI use was associated with a better prognosis even in a shorter period.
The strength of this study lies in the fact that it was conducted in a real-world clinical setting and compared several advanced lung cancer patients to determine whether the addition of ICI increases the risk of thromboembolism, using a platinum-based chemotherapy arm as a control: we showed that the risk of venous thrombosis is approximately 1.3-fold higher with the addition of ICIs. Furthermore, instrumental variable methods were used to adjust for unmeasured confounding, and the results showed a similar trend, indicating that the results are robust. We believe that the current study may provide important information in the decision-making process for lung cancer treatment. However, this study had some limitations. First, outpatient treatment data were unavailable. In Japan, most platinum combination chemotherapies are initiated in an inpatient setting [24]. Therefore, the first administration of the target regimen could be identified using inpatient data only. Second, the unavailability of echocardiographic and electrocardiographic results and information on programmed cell death-ligand 1 antibodies could have led to a bias in the treatment choice to administer ICI. Furthermore, information on histological types (squamous cell carcinoma, adenocarcinoma) and oncogenic gene mutations could not be obtained from the database [48]. In lung and esophageal cancer, the risk of VTE has been reported to be higher for adenocarcinoma than for squamous cell carcinoma [49, 50]. Oncogenic gene mutations, especially ALK fusion gene mutations, are known risk factors for thrombosis [51]. However, the instrumental variable method can adjust for such unmeasured confounders and would support the results of the main analyses. Third, information on outcomes outside the hospital (e.g., thromboembolisms treated in other hospitals and death at home) could not be obtained. Mild venous thrombosis without inpatient treatment may also have been missed. Hence, there may be an underestimation of the outcomes. Moreover, angina pectoris treated only with antiplatelet agents was not included as an outcome. However, because the lack of information is expected to occur equally in the two groups, they are not likely to skew the results. Fourth, with the approval of direct oral anticoagulants, the detection of thrombosis in the ICI and non-ICI groups may be different. For example, in Trousseau syndrome, heparin and direct oral anticoagulants are more likely to be used and warfarin is less frequently selected. Patients with ischemic brain disease in the non-ICI group before direct oral anticoagulants approval may not have been treated with warfarin and thus may have been undetected. Finally, since patients with a history of thromboembolism and patients already taking anticoagulants were excluded, further investigation on this population is needed.
Conclusion
Adding ICIs to platinum combination chemotherapy was associated with an increased risk of in-hospital VTE within 6 months after the start of platinum combination chemotherapy compared with platinum combination chemotherapy alone, while the risk of in-hospital ATE was similar. Therefore, clinicians should closely monitor patients for the risk of thromboembolism when using regimens with ICI added to platinum combination chemotherapy.
Data availability
The data analyzed during the current study are not publicly available due to contracts with the hospitals providing data to the database. Further inquiries on data can be directed to the corresponding author.
References
Grilz E, Königsbrügge O, Posch F et al (2018) Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica 103:1549–1556. https://doi.org/10.3324/haematol.2018.192419
Cohen AT, Katholing A, Rietbrock S et al (2017) Epidemiology of first and recurrent venous thromboembolism in patients with active cancer a population-based cohort study. Thromb Haemost 117:57–65. https://doi.org/10.1160/TH15-08-0686
Ay C, Pabinger I, Cohen AT (2017) Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost 117:219–230. https://doi.org/10.1160/TH16-08-0615
Nalluri SR, Chu D, Keresztes R et al (2008) Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 300:2277–2285. https://doi.org/10.1001/jama.2008.656
Totzeck M, Mincu RI, Rassaf T (2017) Cardiovascular adverse events in patients with cancer treated with bevacizumab: a meta-analysis of more than 20 000 patients. J Am Heart Assoc 6:e006278. https://doi.org/10.1161/JAHA.117.006278
Bar J, Markel G, Gottfried T et al (2019) Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer 120:122–131. https://doi.org/10.1016/j.ejca.2019.06.021
Gong J, Drobni ZD, Alvi RM et al (2021) Immune checkpoint inhibitors for cancer and venous thromboembolic events. Eur J Cancer 158:99–110. https://doi.org/10.1016/j.ejca.2021.09.010
Moik F, Chan W-SE, Wiedemann S et al (2021) Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood 137:1669–1678. https://doi.org/10.1182/blood.2020007878
Ando Y, Hayashi T, Sugimoto R et al (2020) Risk factors for cancer-associated thrombosis in patients undergoing treatment with immune checkpoint inhibitors. Invest New Drugs 38:1200–1206. https://doi.org/10.1007/s10637-019-00881-6
Deschênes-Simard X, Richard C, Galland L et al (2021) Venous thrombotic events in patients treated with immune checkpoint inhibitors for non-small cell lung cancer: a retrospective multicentric cohort study. Thromb Res 205:29–39. https://doi.org/10.1016/j.thromres.2021.06.018
Urwyler P, Earnshaw I, Bermudez M et al (2020) Mechanisms of checkpoint inhibition-induced adverse events. Clin Exp Immunol 200:141–154. https://doi.org/10.1111/cei.13421
Esmon CT (2003) Inflammation and thrombosis. J Thromb Haemost 1:1343–1348. https://doi.org/10.1046/j.1538-7836.2003.00261.x
Franco AT, Corken A, Ware J (2015) Platelets at the interface of thrombosis, inflammation, and cancer. Blood 126:582–588. https://doi.org/10.1182/blood-2014-08-531582
Engelmann B, Massberg S (2013) Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 13:34–45. https://doi.org/10.1038/nri3345
Khorana AA, Palaia J, Rosenblatt L et al (2023) Venous thromboembolism incidence and risk factors associated with immune checkpoint inhibitors among patients with advanced non-small cell lung cancer. J Immunother Cancer 11:e006072. https://doi.org/10.1136/jitc-2022-006072
Drobni ZD, Alvi RM, Taron J et al (2020) Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 142:2299–2311. https://doi.org/10.1161/CIRCULATIONAHA.120.049981
Giustozzi M, Becattini C, Roila F et al (2021) Vascular events with immune checkpoint inhibitors in melanoma or non-small cell lung cancer: a systematic review and meta-analysis. Cancer Treat Rev 100:102280. https://doi.org/10.1016/j.ctrv.2021.102280
Yamana H, Moriwaki M, Horiguchi H et al (2017) Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 27:476–482. https://doi.org/10.1016/j.je.2016.09.009
Yasunaga H (2019) Real world data in Japan: chapter II the diagnosis procedure combination database. Ann Clin Epidemiol 1:76–79
Iwai C, Jo T, Konishi T et al (2023) Comparative safety and effectiveness of direct oral anticoagulants and warfarin during chemotherapy in cancer patients with venous thromboembolism aged 75 years or older: a nationwide inpatient database study. Gerontology 69:561–570. https://doi.org/10.1159/000528606
Katano S, Yano T, Ohori K et al (2022) Barthel index score predicts mortality in elderly heart failure–a goal of comprehensive cardiac rehabilitation. Circ J 86:70–78. https://doi.org/10.1253/circj.CJ-21-0584
Konishi T, Sakata A, Inokuchi H et al (2022) Treatments and outcomes of adult parapharyngeal and retropharyngeal abscess: 1882 cases from a Japanese nationwide database. Am J Otolaryngol 44:103770. https://doi.org/10.1016/j.amjoto.2022.103770
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Mitani A, Jo T, Yasunaga H et al (2018) Venous thromboembolic events in patients with lung cancer treated with cisplatin-based versus carboplatin/nedaplatin-based chemotherapy. Anticancer Drugs 29:560–564. https://doi.org/10.1097/CAD.0000000000000625
Desai RJ, Franklin JM (2019) Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 367:l5657. https://doi.org/10.1136/bmj.l5657
Thomas LE, Li F, Pencina MJ (2020) Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA 323:2417–2418. https://doi.org/10.1001/jama.2020.7819
Mehta N, Kalra A, Nowacki AS et al (2020) Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol 5:1020–1026. https://doi.org/10.1001/jamacardio.2020.1855
**an Y, Xu H, O’Brien EC et al (2019) Clinical effectiveness of direct oral anticoagulants vs warfarin in older patients with atrial fibrillation and ischemic stroke: findings from the patient-centered research into outcomes stroke patients prefer and effectiveness research (PROSPER) study. JAMA Neurol 76:1192–1202. https://doi.org/10.1001/jamaneurol.2019.2099
Li F, Morgan KL, Zaslavsky AM (2018) Balancing covariates via propensity score weighting. J Am Stat Assoc 113:390–400. https://doi.org/10.1080/01621459.2016.1260466
Li F, Thomas LE, Li F (2019) Addressing extreme propensity scores via the overlap weights. Am J Epidemiol 188:250–257. https://doi.org/10.1093/aje/kwy201
Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083–3107. https://doi.org/10.1002/sim.3697
Noordzij M, Leffondré K, van Stralen KJ et al (2013) When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 28:2670–2677. https://doi.org/10.1093/ndt/gft355
Austin PC, Lee DS, Fine JP (2016) Introduction to the analysis of survival data in the presence of competing risks. Circulation 133:601–609. https://doi.org/10.1161/CIRCULATIONAHA.115.017719
Froesch P, Martucci F, Györik S et al (2014) Management of non-small cell lung cancer in the elderly. Eur J Intern Med 25:888–894. https://doi.org/10.1016/j.ejim.2014.10.024
Fujimoto D, Miura S, Yoshimura K et al (2022) A real-world study on the effectiveness and safety of pembrolizumab plus chemotherapy for nonsquamous NSCLC. JTO Clin Res Rep 3:100265. https://doi.org/10.1016/j.jtocrr.2021.100265
Morimoto K, Yamada T, Yokoi T et al (2021) Clinical impact of pembrolizumab combined with chemotherapy in elderly patients with advanced non-small-cell lung cancer. Lung Cancer 161:26–33. https://doi.org/10.1016/j.lungcan.2021.08.015
Newhouse JP, McClellan M (1998) Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health 19:17–34. https://doi.org/10.1146/annurev.publhealth.19.1.17
Brookhart MA, Rassen JA, Schneeweiss S (2010) Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf 19:537–554. https://doi.org/10.1002/pds.1908
Greenland S (2000) An introduction to instrumental variables for epidemiologists. Int J Epidemiol 29:1102. https://doi.org/10.1093/oxfordjournals.ije.a019909
Baiocchi M, Cheng J, Small DS (2014) Tutorial in biostatistics: instrumental variable methods for causal inference*. Stat Med 33:2297–2340. https://doi.org/10.1002/sim.6128
Champiat S, Lambotte O, Barreau E et al (2016) Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 27:559–574. https://doi.org/10.1093/annonc/mdv623
Brahmer JR, Lacchetti C, Schneider BJ et al (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 36:1714–1768. https://doi.org/10.1200/JCO.2017.77.6385
Dugbartey GJ, Peppone LJ, de Graaf IAM (2016) An integrative view of cisplatin-induced renal and cardiac toxicities: molecular mechanisms, current treatment challenges and potential protective measures. Toxicology 371:58–66. https://doi.org/10.1016/j.tox.2016.10.001
Paz-Ares L, Vicente D, Tafreshi A et al (2020) A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 15:1657–1669. https://doi.org/10.1016/j.jtho.2020.06.015
West H, McCleod M, Hussein M et al (2019) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:924–937. https://doi.org/10.1016/S1470-2045(19)30167-6
Nishio M, Barlesi F, West H et al (2021) Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 Trial. J Thorac Oncol 16:653–664. https://doi.org/10.1016/j.jtho.2020.11.025
Gadgeel S, Rodríguez-Abreu D, Speranza G et al (2020) Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 38:1505–1517. https://doi.org/10.1200/JCO.19.03136
Hirano Y, Kaneko H, Konishi T et al (2022) ASO author reflections: epidural analgesia decreases in-hospital mortality, respiratory complications, and anastomotic leakage after minimally invasive esophagectomy for esophageal cancer. Ann Surg Oncol 29:8235–8236. https://doi.org/10.1245/s10434-022-12431-1
Blom JW, Osanto S, Rosendaal FR (2004) The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost 2:1760–1765. https://doi.org/10.1111/j.1538-7836.2004.00928.x
Akhtar-Danesh G-G, Akhtar-Danesh N, Shargall Y (2022) Venous thromboembolism in surgically treated esophageal cancer patients: a provincial population-based study. TH Open 6:e168–e176. https://doi.org/10.1055/s-0042-1750378
Wang H-Y, Wu S-G, Lin Y-T et al (2022) Risk of thromboembolism in non-small-cell lung cancers patients with different oncogenic drivers, including ROS1, ALK, and EGFR mutations. ESMO Open 7:100742. https://doi.org/10.1016/j.esmoop.2022.100742
Funding
Open access funding provided by The University of Tokyo. This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (23AA2003 and 22AA2003).
Author information
Authors and Affiliations
Contributions
C. I designed the study, analyzed and interpreted the data, and prepared the manuscript. T. J designed the study, analyzed and interpreted the data, and prepared the manuscript. T. K analyzed, interpreted the data, and prepared the manuscript. A. F collected and interpreted the data. N. M analyzed and interpreted the data, and H. M collected and interpreted the data. K. F collected and interpreted the data. H. Y designed the study, collected and interpreted the data, and prepared the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethical statement
This study was approved by the Institutional Review Board of the University of Tokyo, which waived the requirement for informed consent because of the anonymity of the patient database (approval number: 3501– (3)). This research was conducted in accordance with the principles of the Declaration of Helsinki and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iwai, C., Jo, T., Konishi, T. et al. Thrombotic risk of platinum combination chemotherapy with and without immune checkpoint inhibitors for advanced non-small cell lung cancer: a nationwide inpatient database study. Cancer Immunol Immunother 72, 3581–3591 (2023). https://doi.org/10.1007/s00262-023-03508-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03508-1