Abstract
Background
In Japan, nivolumab administration is the standard treatment for patients with unresectable advanced or recurrent esophageal squamous cell carcinoma (ESCC) who are refractory or intolerant to fluoropyrimidines and platinum-based chemotherapy. We determined if inflammatory prognostic factors are useful in patients with ESCC treated with nivolumab monotherapy.
Methods
The clinical data of patients with ESCC treated with nivolumab monotherapy as the second- or later-line treatment were retrospectively analyzed. Neutrophil/lymphocyte, platelet/lymphocyte, and C-reactive protein/albumin ratios (CAR); prognostic index; and prognostic nutritional index were investigated. Cut-off values for each factor were determined according to overall survival using time-dependent receiver operating characteristic curves.
Results
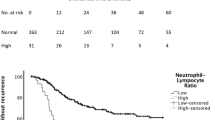
During January 2017–June 2021, 93 consecutive patients with ESCC were enrolled from five institutions (median age, 70 years; male, 77%). With a median follow-up period of 9.1 (range, 1.0–34.7) months, the median overall and progression-free survival were 12.8 (95% confidence interval [CI], 9.0–16.6) and 4.0 (95% CI, 2.6–5.4) months, respectively. Of five inflammatory prognostic factors, the cut-off value for CAR was 0.62; prognosis was significantly longer in those with CAR < 0.62 (hazard ratio, 0.39; 95% CI, 0.22–0.67; p = 0.001).
Conclusions
Inflammatory prognostic factors were useful in predicting prognosis for ESCC patients pretreated with nivolumab, especially for those with CAR < 0.62, suggesting that CAR adequately reflects prognosis.


Similar content being viewed by others
References
Napier KJ, Scheerer M, Misra S (2014) Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 6:112–120. https://doi.org/10.4251/wjgo.v6.i5.112
Koizumi S, Motoyama S, Iijima K (2018) Is the incidence of esophageal adenocarcinoma increasing in Japan? Trends from the data of a hospital-based registration system in Akita Prefecture, Japan. J Gastroenterol 53:827–833. https://doi.org/10.1007/s00535-017-1412-4
National comprehensive cancer network clinical practice guidelines in oncology esophageal and esophagogastric junction cancers version 2.2021.
Hayashi K, Ando N, Watanabe H, Ide H, Nagai K, Aoyama N, Takiyama W, Ishida K, Isono K, Makuuchi H, Imamura M, Shinoda M, Ikeuchi S, Kabuto T, Yamana H, Fukuda H (2001) Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan esophageal oncology group (JEOG) Trial (JCOG9407). Jpn J Clin Oncol 31:419–423. https://doi.org/10.1093/jjco/hye090
Iizuka T, Kakegawa T, Ide H, Ando N, Watanabe H, Tanaka O, Takagi I, Isono K, Ishida K, Arimori M et al (1992) Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese esophageal oncology group trial. Jpn J Clin Oncol 22:172–176
Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y (2019) Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:1506–1517. https://doi.org/10.1016/s1470-2045(19)30626-6
Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Shen L, Enzinger P, Qin SK, Ferreira P, Chen J, Girotto G, de la Fouchardiere C, Senellart H, Al-Rajabi R, Lordick F, Wang R, Suryawanshi S, Bhagia P, Kang SP, Metges JP, KEYNOTE-181 Investigators, (2020) Randomized Phase III KEYNOTE-181 Study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 38:4138–4148. https://doi.org/10.1200/jco.20.01888
Laird BJ, Kaasa S, McMillan DC, Fallon MT, Hjermstad MJ, Fayers P (2013) Klepstad P (2013) Prognostic factors in patients with advanced cancer: a comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Cancer Res 19:5456–5464. https://doi.org/10.1158/1078-0432.Ccr-13-1066
Hiam-Galvez KJ, Allen BM, Spitzer MH (2021) Systemic immunity in cancer. Nat Rev Cancer 21:345–359. https://doi.org/10.1038/s41568-021-00347-z
Kudou K, Nakashima Y, Haruta Y, Nambara S, Tsuda Y, Kusumoto E, Ando K, Kimura Y, Hashimoto K, Yoshinaga K, Saeki H, Oki E, Sakaguchi Y, Kusumoto T, Ikejiri K, Shimokawa M, Mori M (2021) Comparison of inflammation-based prognostic scores associated with the prognostic impact of adenocarcinoma of esophagogastric junction and upper gastric cancer. Ann Surg Oncol 28:2059–2067. https://doi.org/10.1245/s10434-020-08821-y
Wei XL, Wang FH, Zhang DS, Qiu MZ, Ren C, ** Y, Zhou YX, Wang DS, He MM, Bai L, Wang F, Luo HY, Li YH, Xu RH (2015) A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer 15:350. https://doi.org/10.1186/s12885-015-1379-6
Kasymjanova G, MacDonald N, Agulnik JS, Cohen V, Pepe C, Kreisman H, Sharma R, Small D (2010) The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol 17:52–58. https://doi.org/10.3747/co.v17i4.567
Liao G, Zhao Z, Yang H, Chen M, Li X (2020) Can prognostic nutritional index be a prediction factor in esophageal cancer?: A meta-analysis. Nutr Cancer 72:187–193. https://doi.org/10.1080/01635581.2019.1631859
Tanoue K, Tamura S, Kusaba H, Shinohara Y, Ito M, Tsuchihashi K, Shirakawa T, Otsuka T, Ohmura H, Isobe T, Ariyama H, Koreishi S, Matsushita Y, Shimokawa H, Tanaka R, Mitsugi K, Akashi K, Baba E (2021) Predictive impact of C-reactive protein to albumin ratio for recurrent or metastatic head and neck squamous cell carcinoma receiving nivolumab. Sci Rep 11:2741. https://doi.org/10.1038/s41598-021-82448-1
Araki T, Tateishi K, Sonehara K, Shinohara Y, Ito M, Tsuchihashi K, Shirakawa T, Otsuka T, Ohmura H, Isobe T, Ariyama H, Koreishi S, Matsushita Y, Shimokawa H, Tanaka R, Mitsugi K, Akashi K, Baba E (2021) Clinical utility of the C-reactive protein:albumin ratio in non-small cell lung cancer patients treated with nivolumab. Thorac Cancer 12:603–612. https://doi.org/10.1111/1759-7714.13788
Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, Simeone E, Mangana J, Schilling B, Di Giacomo AM, Brenner N, Kähler K, Heinzerling L, Gutzmer R, Bender A, Gebhardt C, Romano E, Meier F, Martus P, Maio M, Blank C, Schadendorf D, Dummer R, Ascierto PA, Hospers G, Garbe C, Wolchok JD (2016) Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 22:5487–5496. https://doi.org/10.1158/1078-0432.CCR-16-0127
Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, Felip E, Zerón-Medina J, Garrido P, Brosseau S, Zalcman G, Mazieres J, Caramela C, Lahmar J, Adam J, Chaput N, Soria JC, Besse B (2018) Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol 4:351–357. https://doi.org/10.1001/jamaoncol.2017.4771
Lalani AA, **e W, Martini DJ, Steinharter JA, Norton CK, Krajewski KM, Duquette A, Bossé D, Bellmunt J, Van Allen EM, McGregor BA, Creighton CJ, Harshman LC, Choueiri TK (2018) Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer 6:5. https://doi.org/10.1186/s40425-018-0315-0
Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, Qian X, Li Y (2020) Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother 69:1813–1822. https://doi.org/10.1007/s00262-020-02585-w
Ogata T, Satake H, Ogata M, Hatachi Y, Inoue K, Hamada M, Yasui H (2018) Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget 9(77):34520
Ota Y, Takahari D, Suzuki T, Osumi H, Nakayama I, Oki A, Wakatsuki T, Ichimura T, Ogura M, Shinozaki E, Suenaga M, Chin K, Yamaguchi K (2020) Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother Pharmacol 85:265–272. https://doi.org/10.1007/s00280-019-04023-w
Guo JC, Lin CC, Lin CY, Hsieh MS, Kuo HY, Lien MY, Hsu CH (2019) Neutrophil–to–lymphocyte ratio and use of antibiotics associated with prognosis in esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitors. Anticancer Res 39(10):5675–5682
Fairclough E, Cairns E, Hamilton J, Kelly C (2009) Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med (Lond) 9:30–33. https://doi.org/10.7861/clinmedicine.9-1-30
Kudou K, Saeki H, Nakashima Y, Kamori T, Kawazoe T, Haruta Y, Fujimoto Y, Matsuoka H, Sasaki S, Jogo T, Hirose K, Hu Q, Tsuda Y, Kimura K, Ando K, Oki E, Ikeda T, Maehara Y (2019) C-reactive protein/albumin ratio is a poor prognostic factor of esophagogastric junction and upper gastric cancer. J Gastroenterol Hepatol 34:355–363. https://doi.org/10.1111/jgh.14442
Matsunaga T, Miyata H, Sugimura K, Motoori M, Asukai K, Yanagimoto Y, Yano M (2019) Prognostic significance of sarcopenia and systemic inflammatory response in patients with esophageal cancer. Anticancer Res 39(1):449–458
Tamagawa H, Aoyama T, Tamagawa A, Komori K, Maezawa Y, Kano K, Rino Y (2020) Influence of the preoperative C-reactive protein-to-albumin ratio on survival and recurrence in patients with esophageal cancer. Anticancer Res 40(4):2365–2371
Ni XF, Wu J, Ji M, Shao YJ, Xu B, Jiang JT, Wu CP (2018) Effect of C-reactive protein/albumin ratio on prognosis in advanced non-small-cell lung cancer. Asia Pac J Clin Oncol 14:402–409. https://doi.org/10.1111/ajco.13055
McKeown DJ, Brown DJ, Kelly A, Wallace AM, McMillan DC (2004) The relationship between circulating concentrations of C-reactive protein, inflammatory cytokines and cytokine receptors in patients with non-small-cell lung cancer. Br J Cancer 91:1993–1995. https://doi.org/10.1038/sj.bjc.6602248
Ara T, Declerck YA (2010) Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer 46:1223–1231. https://doi.org/10.1016/j.ejca.2010.02.026
Foran E, Garrity-Park MM, Mureau C, Newell J, Smyrk TC, Limburg PJ, Egan LJ (2010) Upregulation of DNA methyltransferase-mediated gene silencing, anchorage-independent growth, and migration of colon cancer cells by interleukin-6. Mol Cancer Res 8:471–481. https://doi.org/10.1158/1541-7786.MCR-09-0496
Middleton K, Jones J, Lwin Z, Coward JI (2014) Interleukin-6: An angiogenic target in solid tumours. Crit Rev Oncol Hematol 89:129–139. https://doi.org/10.1016/j.critrevonc.2013.08.004
Fujiwara H, Suchi K, Okamura S, Okamura H, Umehara S, Todo M, Shiozaki A, Kubota T, Ichikawa D, Okamoto K, Ochiai T, Kokuba Y, Sonoyama T, Otsuji E (2011) Elevated serum CRP levels after induction chemoradiotherapy reflect poor treatment response in association with IL-6 in serum and local tumor site in patients with advanced esophageal cancer. J Surg Oncol 103:62–68. https://doi.org/10.1002/jso.21751
Wigmore SJ, Fearon KC, Maingay JP, Lai PB, Ross JA (1997) Interleukin-8 can mediate acute-phase protein production by isolated human hepatocytes. Am J Physiol 273:E720-726. https://doi.org/10.1152/ajpendo.1997.273.4.E720
Liu Z, Shi H, Chen L (2019) Prognostic role of pre-treatment C-reactive protein/albumin ratio in esophageal cancer: a meta-analysis. BMC Cancer 19:1161. https://doi.org/10.1186/s12885-019-6373-y
Takahashi M, Kato K, Okada M, Chin K, Kadowaki S, Hamamoto Y, Doki Y, Kubota Y, Kawakami H, Ogata T, Hara H, Muto M, Nakashima Y, Ishihara R, Tsuda M, Motoyama S, Kodani M, Kitagawa Y (2021) Nivolumab versus chemotherapy in Japanese patients with advanced esophageal squamous cell carcinoma: a subgroup analysis of a multicenter, randomized, open-label, phase 3 trial (ATTRACTION-3). Esophagus 18:90–99. https://doi.org/10.1007/s10388-020-00794-x
Acknowledgements
I am deeply grateful to Prof. Satake and the other co-authors for their guidance. We also thank Editage, which was responsible for English editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Tatsuki Ikoma, Hironaga Satake, Mototsugu Shimokawa, Shogen Boku and other authors were involved in investigation, data curation, writing—review & editing and visualization. Tatsuki Ikoma, Hironaga Satake and Shogen Boku were involved in writing—original draft. Tatsuki Ikoma and Hironaga Satake performed conceptualization and methodology. Hironaga Satake was involved in supervision and project administration. Tatsuki Ikoma, Hironaga Satake and Mototsugu Shimokawa were involved in formal analysis.
Corresponding author
Ethics declarations
Conflict of interest
Hironaga Satake received research funding from Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd., and honoraria from Bayer, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Eli Lilly Japan, Merck Bio Pharma, MSD, Ono Pharmaceutical, Sanofi, Taiho Pharmaceutical Co., Ltd., Takeda, and Yakult Honsha. However, those funding sources had no involvement with our study. The rest of the authors do not have any conflict of interest.
Ethics approval
This study was conducted in accordance with the Helsinki Declaration of 1964 and later versions, and with the Ethical Guidelines for Clinical Studies. This study was approved by the institutional review boards of all three participating institutions: Kobe City Medical Center General Hospital (approval no. zn210501), Kansai Medical University Hospital, Kochi Medical School, Himeji Red Cross Hospital, and Osaka Red Cross hospital.
Consent to participate
The requirement for informed consent was waived owing to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikoma, T., Shimokawa, M., Matsumoto, T. et al. Inflammatory prognostic factors in advanced or recurrent esophageal squamous cell carcinoma treated with nivolumab. Cancer Immunol Immunother 72, 427–435 (2023). https://doi.org/10.1007/s00262-022-03265-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03265-7