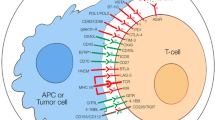
Abstract
Cancer immunotherapy, which blocks immune checkpoint molecules, is an effective therapeutic strategy for human cancer patients through restoration of tumor-infiltrating (TI) cell function. However, evaluating the efficacy of immune checkpoint inhibitors (ICIs) is difficult because no standard in vitro assay for ICI efficacy evaluation exists. Additionally, blocking a particular immune checkpoint receptor (ICR) is insufficient to restore T cell functionality, because other ICRs still transduce inhibitory signals. Therefore, limiting inhibitory signals transduced via other ICRs is needed to more accurately assess the efficacy of ICIs targeting a particular immune checkpoint. Here, we introduce a newly developed in vitro coculture assay using human peripheral blood mononuclear cells (hPBMCs) and engineered human cancer cell lines. We enriched CD8+ T cells from hPBMCs of healthy donors through low-dose T cell receptor stimulation and cytokine (human IL-2 and IL-7) addition. These enriched CD8+ T cells were functional and expressed multiple ICRs, especially TIM-3 and TIGIT. We also established immune checkpoint ligand (ICL) knockout (KO) cancer cell lines with the CRISPR-Cas9 system. Then, we optimized the in vitro coculture assay conditions to evaluate ICI efficacy. For example, we selected the most effective anti-TIM-3 antibody through coculture of TIM-3+CD8+ T cells with PD-L1-/-PVR-/- cancer cells. In summary, we developed a mechanism-based in vitro coculture assay with hPBMCs and ICL KO cancer cell lines, which could be a useful tool to identify promising ICIs by providing reliable ICI efficacy information.







Similar content being viewed by others
Data availability
All data generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- Cas9:
-
CRISPR-associated protein 9
- hPBMC:
-
Human peripheral blood mononuclear cell
- ICI:
-
Immune checkpoint inhibitor
- ICL:
-
Immune checkpoint ligand
- ICR:
-
Immune checkpoint receptor
- TI:
-
Tumor-infiltrating
References
Pauken KE, Wherry EJ (2015) Overcoming T cell exhaustion in infection and cancer. Trends Immunol 36:265–276. https://doi.org/10.1016/j.it.2015.02.008
Anderson AC, Joller N, Kuchroo VK (2016) Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 44:989–1004. https://doi.org/10.1016/j.immuni.2016.05.001
Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM (2010) Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 207:2175–2186. https://doi.org/10.1084/jem.20100637
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P et al (2010) Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci 107:7875–7880. https://doi.org/10.1073/pnas.1003345107
Chauvin JM, Pagliano O, Fourcade J et al (2015) TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest 125:2046–2058. https://doi.org/10.1172/JCI80445
Kamphorst AO, Pillai RN, Yang S et al (2017) Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA 114:4993–4998. https://doi.org/10.1073/pnas.1705327114
Thommen DS, Koelzer VH, Herzig P et al (2018) A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 24:994–1004. https://doi.org/10.1038/s41591-018-0057-z
Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AMM, Redmond WL, Seliger B, Marincola FM (2017) Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer 81:116–129. https://doi.org/10.1016/j.ejca.2017.01.035
Chen L, Han X (2015) Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 125:3384–3391. https://doi.org/10.1172/JCI80011
Gong J, Chehrazi-Raffle A, Reddi S, Salgia R (2018) Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer 6:8. https://doi.org/10.1186/s40425-018-0316-z
Kim JE, Patel MA, Mangraviti A et al (2017) Combination therapy with anti-PD-1, Anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res 23:124–136. https://doi.org/10.1158/1078-0432.CCR-15-1535
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207:2187–2194. https://doi.org/10.1084/jem.20100643
Hung AL, Maxwell R, Theodros D et al (2018) TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology 7:e1466769. https://doi.org/10.1080/2162402X.2018.1466769
Harris-Bookman S, Mathios D, Martin AM et al (2018) Expression of LAG-3 and efficacy of combination treatment with anti-LAG-3 and anti-PD-1 monoclonal antibodies in glioblastoma. Int J Cancer 143:3201–3208. https://doi.org/10.1002/ijc.31661
Yonesaka K, Haratani K, Takamura S et al (2018) B7–H3 negatively modulates CTL-mediated cancer immunity. Clin Cancer Res 24:2653–2664. https://doi.org/10.1158/1078-0432.CCR-17-2852
Lee YH, Martin-Orozco N, Zheng P et al (2017) Inhibition of the B7–H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res 27:1034–1045. https://doi.org/10.1038/cr.2017.90
Rotte A (2019) Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res 38:255. https://doi.org/10.1186/s13046-019-1259-z
Kamphorst AO, Wieland A, Nasti T et al (2017) Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355:1423–1427. https://doi.org/10.1126/science.aaf0683
Hui E, Cheung J, Zhu J et al (2017) T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355:1428–1433. https://doi.org/10.1126/science.aaf1292
Khan O, Giles JR, McDonald S et al (2019) TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature 571:211–218. https://doi.org/10.1038/s41586-019-1325-x
Han HS, Jeong S, Kim H et al (2021) TOX-expressing terminally exhausted tumor-infiltrating CD8(+) T cells are reinvigorated by co-blockade of PD-1 and TIGIT in bladder cancer. Cancer Lett 499:137–147. https://doi.org/10.1016/j.canlet.2020.11.035
Kim K, Park S, Park SY et al (2020) Single-cell transcriptome analysis reveals TOX as a promoting factor for T cell exhaustion and a predictor for anti-PD-1 responses in human cancer. Genome Med 12:22. https://doi.org/10.1186/s13073-020-00722-9
Sharpe AH, Pauken KE (2018) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18:153–167. https://doi.org/10.1038/nri.2017.108
Kim KH, Cho J, Ku BM et al (2019) The first-week proliferative response of peripheral blood PD-1(+)CD8(+) T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res 25:2144–2154. https://doi.org/10.1158/1078-0432.CCR-18-1449
Kim KH, Hur JY, Koh J et al (2020) Immunological characteristics of hyperprogressive disease in patients with non-small cell lung cancer treated with anti-PD-1/PD-L1 abs. Immune Netw 20:e48. https://doi.org/10.4110/in.2020.20.e48
Abraham RT, Weiss A (2004) Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol 4:301–308. https://doi.org/10.1038/nri1330
Wang C, Thudium KB, Han M et al (2014) In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2:846–856. https://doi.org/10.1158/2326-6066.CIR-14-0040
Kwon M, Choi YJ, Sa M, Park SH, Shin EC (2018) Two-round mixed lymphocyte reaction for evaluation of the functional activities of Anti-PD-1 and Immunomodulators. Immune Netw 18:e45. https://doi.org/10.4110/in.2018.18.e45
Mujib S, Jones RB, Lo C, Aidarus N, Clayton K, Sakhdari A, Benko E, Kovacs C, Ostrowski MA (2012) Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. J Immunol 188:3745–3756. https://doi.org/10.4049/jimmunol.1102609
Zhang ZN, Zhu ML, Chen YH, Fu YJ, Zhang TW, Jiang YJ, Chu ZX, Shang H (2015) Elevation of Tim-3 and PD-1 expression on T cells appears early in HIV infection, and differential Tim-3 and PD-1 expression patterns can be induced by common gamma -chain cytokines. Biomed Res Int 2015:916936. https://doi.org/10.1155/2015/916936
Martin MD, Badovinac VP (2018) Defining memory CD8 T cell. Front Immunol 9:2692. https://doi.org/10.3389/fimmu.2018.02692
Picarda E, Ohaegbulam KC, Zang X (2016) Molecular pathways: targeting B7–H3 (CD276) for human cancer immunotherapy. Clin Cancer Res 22:3425–3431. https://doi.org/10.1158/1078-0432.CCR-15-2428
Funding
The authors declare no competing financial interests. This study was supported by grants funded by the Ministry of Food and Drug Safety (18182MFDS408) and the Ministry of Science and ICT (MSIT) (2017R1A5A1014560, 2019M3A9B6065221). This study was also supported by Korean Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare (HV20C0144) and Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (HN21C1410). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
MJK and S-JH designed and interpreted the study, wrote the manuscript and edited the manuscript. MJK performed the experiments and analyzed the data. KHH and BRL assisted with experiments. S-JH supervised the study. All authors approved the final version of the article, including the authorship list.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Ethical approval
The studies were approved by the Institutional Review Board of Yonsei University Severance Hospital with IRB no. 4-2016-0788 for a single patient with NSCLC and a single patient with HNSCC. All patients who participated in these studies provided written informed consent prior to enrollment and sample collection at Yonsei University Severance Hospital. The research conformed to the principles of the Helsinki Declaration.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
262_2022_3201_MOESM1_ESM.pdf
Supplementary Figure 1 Comparison between the CD8+ T cell enrichments by the existing protocol and the newly developed protocol. hPBMCs were isolated from the peripheral blood of healthy donors. For the existing protocol using αCD3/CD28 dynabeads, isolated hPBMCs were cultured with αCD3/CD28 dynabeads (the ratio of 1:1) and hIL-2 (10 ng/ml). For the newly developed protocol using soluble anti-CD3 antibodies, isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in non-tissue 24-well culture plates. (a) Representative FACS plot of singlets, lymphocytes, live cells and T cells of the cultured hPBMCs for 15 days according to the indicated protocol. The experiment was performed with hPBMCs from three donors. The data are representative of a single donor. (PDF 436 KB)
262_2022_3201_MOESM2_ESM.pdf
Supplementary Figure 2 Comparison between the CD8+ T cell enrichments by using hPBMCs of healthy donors and cancer patients. hPBMCs were isolated from the peripheral blood of healthy donors, a single patient with NSCLC, and a single patient with HNSCC. Isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in non-tissue 24-well culture plates. (a) Representative FACS plot of the T cell frequency at the indicated time point after culture initiation. (b) Kinetics of the number of total cells among the cultured hPBMCs. (c) Kinetics of the frequency of T cells, and the number of T cells among the cultured hPBMCs. The experiment was performed with hPBMCs from healthy donors, a single patient with NSCLC, and a single patient with HNSCC. (PDF 332 KB)
262_2022_3201_MOESM3_ESM.pdf
Supplementary Figure 3 Naïve/memory phenotype of enriched CD8+ T cells. hPBMCs were isolated from the peripheral blood of healthy donors. Isolated hPBMCs were cultured with anti-CD3 antibodies (1 μg/ml, soluble), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml) in non-tissue 24-well culture plates. (a) Representative FACS plot of naïve/memory phenotype of cultured CD8+ T cells at the indicated time point after culture initiation. (b) Quantification of the percentage of effector memory (EM), effector memory expressing CD45RA (EMRA), central memory (CM), and naïve CD8+ T cells at the indicated time point after culture initiation. (c) Representative FACS plot of the ICR expression patterns of CD8+ T cells enriched 15 days according to naïve/memory phenotype. (d) Representative FACS plot of naïve/memory CD8+ T cell frequency in PD-1+ and PD-1-CD8+ T cells at 15 days after culture initiation (left). Quantification of the percentage of naïve/memory CD8+ T cell frequency in PD-1+ and PD-1-CD8+ T cells at 15 days after culture initiation (right). The experiment was performed with hPBMCs from two donors. The data are representative of triplicate samples from a single donor. The data were analyzed by two-tailed unpaired Student’s t-test (b, d). The error bars indicate the means ± SEMs. **p < 0.01; ****p < 0.0001. (PDF 938 KB)
262_2022_3201_MOESM4_ESM.pdf
Supplementary Figure 4 Degranulation in ICR-positive CD8+ T cells was augmented by ICIs. Before coculture, CD8+ T cells enriched for 21 days were preincubated with isotype control or ICR-blocking antibodies for 20 min in a 37°C incubator. Preincubated CD8+ T cells were cocultured with HCC4006 cell lines for 6 hr at a ratio of 1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). (a) Representative FACS plots of cytokine secretion and degranulation in PD-1+CD8+ T cells cultured with and without anti-PD-1 antibodies. (b) Rate of increase in PD-1+CD8+ T cell degranulation induced by anti-PD-1 antibody treatment. (c) Representative FACS plots of cytokine secretion and degranulation in TIGIT+CD8+ T cells cultured with and without anti-TIGIT antibodies. (d) Rate of increase in TIGIT+CD8+ T cell degranulation induced by anti-TIGIT antibody treatment. The data were concatenated in each group (a, c). The data were analyzed by two-tailed unpaired Student’s t-test (b,d). The error bars indicate the means ± SEMs. **p < 0.01; ****p < 0.0001. (PDF 496 KB)
262_2022_3201_MOESM5_ESM.pdf
Supplementary Figure 5 Comparison between the increased cytokine secretion in TIGIT+CD8+ T cells in the ratio of 1:1 and 0.1:1. Before coculture, CD8+ T cells enriched for 21 days were preincubated with isotype control or TIGIT-blocking antibodies for 20 min in a 37°C incubator. Preincubated CD8+ T cells were cocultured with HCC4006 cell lines for 6 hr at a ratio of 1:1 and 0.1:1 under restimulation with the anti-CD3 antibody (20 μg/ml, plate-coated), the anti-CD28 antibody (5 μg/ml, plate-coated), hIL-2 (10 ng/ml), and hIL-7 (10 ng/ml). (a) Representative FACS plot of cytokine secreting-TIGIT+CD8+ T cells cultured with and without anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. (b) Rate of increase in TIGIT+CD8+ T cell function induced by anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. (c) Representative FACS plot of degranulation in TIGIT+CD8+ T cells cultured with and without anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. (d) Rate of increase in TIGIT+CD8+ T cell degranulation induced by anti-TIGIT antibodies in the ratio of 1:1 and 0.1:1. The data were concatenated in each group (a, c). The data were analyzed by two-tailed unpaired Student’s t-test (b,d). The error bars indicate the means ± SEMs. ***p < 0.001; ****p < 0.0001. (PDF 490 KB)
Rights and permissions
About this article
Cite this article
Kim, M.J., Hong, K.H., Lee, B.R. et al. Establishment of a mechanism-based in vitro coculture assay for evaluating the efficacy of immune checkpoint inhibitors. Cancer Immunol Immunother 71, 2777–2789 (2022). https://doi.org/10.1007/s00262-022-03201-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03201-9