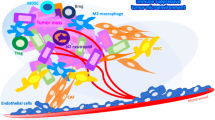
Abstract
Background
Since the lung is one of the most common sites for cancer metastasis, it could provide a suitable microenvironment for pre-metastatic niche (PMN) formation to facilitate tumor cell colonization. Regulatory T cells (Tregs) are an immunosuppressive cell type found ubiquitously in tumors and may play a crucial role in PNM formation. In this study, we investigated tumor-derived exosome (TDE)-induced Treg differentiation in the lung PMN as well as the underlying mechanisms.
Methods
TDEs were isolated from the Lewis lung carcinoma cell line (LLC-exo) and their effects on mouse pulmonary fibroblasts was investigated in vitro as well as on lung tumor formation and metastasis in a pre-injected mouse model. Immune cell populations in the lung were analyzed by flow cytometry. Expression of CCL1 and CCR8 was evaluated by immunofluorescence staining, qRT-PCR and Western blot analyses. Cytokine expression was measured using mouse cytokine arrays and ELISA.
Results
The number of CD4+ FoxP3+ Tregs was significantly increased in lungs in a LLC-exo pre-injected mouse model. Lung fibroblasts secreted increased amounts of CCL1 after co-culture with LLC-exo, which induced Treg differentiation by activating its specific receptor CCR8, ultimately contributing to the establishment of an immunologically tolerant PMN. Moreover, inhibiting the release of LLC-exo by GW4869, or blocking the CCL1-CCR8 axis using AZ084, suppressed Tregs differentiation and tumor metastasis in the lung.
Conclusions
Collectively, our study provides a novel mechanism by which Tregs are activated to form an immunologically tolerant PMN and demonstrates a critical link among lung fibroblasts, Tregs and metastatic tumor cells.







Similar content being viewed by others
Availability of data and materials
The authors declare that all data and materials are available on request.
Abbreviations
- CAF:
-
Cancer-associated fibroblasts
- DMED:
-
Dulbecco’s Modified Eagle’s Medium
- ECM:
-
Extracellular matrix
- EV:
-
Extracellular vesicles
- FBS:
-
Fetal bovine serum
- HE:
-
Hematoxylin and eosin
- LLC-exo:
-
Lewis lung carcinoma exosome
- MDSCs:
-
Myeloid-derived suppressor cells
- MPF:
-
Mouse pulmonary fibroblasts
- PMN:
-
Pre-metastatic niche
- RBC:
-
Red blood cells
- TDE:
-
Tumor cell-derived exosomes
- TME:
-
Tumor microenvironment
- Tregs:
-
Regulatory T cells
References
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331:1559–1564
Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127:679–695
de Groot AE, Roy S, Brown JS, Pienta KJ, Amend SR (2017) Revisiting seed and soil: examining the primary tumor and cancer cell foraging in metastasis. Mol Cancer Res 15:361–370
Liu Q, Zhang H, Jiang X, Qian C, Liu Z, Luo D (2017) Factors involved in cancer metastasis: a better understanding to “seed and soil” hypothesis. Mol Cancer 16:176
Akhtar M, Haider A, Rashid S, Al-Nabet A (2019) Paget’s, “seed and soil” theory of cancer metastasis: an idea whose time has come. Adv Anat Pathol 26:69–74
Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G et al (2017) Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 17:302–317
Liu Y, Cao X (2016) Characteristics and significance of the pre-metastatic niche. Cancer Cell 30:668–681
Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C et al (2019) Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer 18:39
Gillot L, Baudin L, Rouaud L, Kridelka F, Noël A (2021) The pre-metastatic niche in lymph nodes: formation and characteristics. Cell Mol Life Sci 78:5987–6002
Doglioni G, Parik S, Fendt SM (2019) Interactions in the (pre) metastatic niche support metastasis formation. Front Oncol 9:219
Zhuge X, Sun Y, Jiang M, Wang J, Tang F, Xue F et al (2019) Acetate metabolic requirement of avian pathogenic Escherichia coli promotes its intracellular proliferation within macrophage. Vet Res 50:31
Ohue Y, Nishikawa H (2019) Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci 110:2080–2089
Tanaka A, Sakaguchi S (2017) Regulatory T cells in cancer immunotherapy. Cell Res 27:109–118
Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL et al (2009) Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res 69:5996–6004
Wortzel I, Dror S, Kenific CM, Lyden D (2019) Exosome-mediated metastasis: communication from a distance. Dev Cell 49:347–360
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M et al (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527:329–335
Sun W, Ren Y, Lu Z, Zhao X (2020) The potential roles of exosomes in pancreatic cancer initiation and metastasis. Mol Cancer 19:135
Zhang YF, Zhou YZ, Zhang B, Huang SF, Li PP, He XM et al (2019) Pancreatic cancer-derived exosomes promoted pancreatic stellate cells recruitment by pancreatic cancer. J Cancer 10:4397–4407
Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C et al (2021) Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab 33:2040–58.e10
Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J (2009) Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A 106:3794–3799
Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y (2020) Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 5:28
Zhang L, Yu D (2019) Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer 1871:455–468
Olejarz W, Dominiak A, Żołnierzak A, Kubiak-Tomaszewska G, Lorenc T (2020) Tumor-derived exosomes in immunosuppression and immunotherapy. J Immunol Res 2020:6272498
Du C, Duan X, Yao X, Wan J, Cheng Y, Wang Y et al (2020) Tumour-derived exosomal miR-3473b promotes lung tumour cell intrapulmonary colonization by activating the nuclear factor-κB of local fibroblasts. J Cell Mol Med 24:7802–7813
Barsheshet Y, Wildbaum G, Levy E, Vitenshtein A, Akinseye C, Griggs J et al (2017) CCR8(+)FOXp3(+) T(reg) cells as master drivers of immune regulation. Proc Natl Acad Sci U S A 114:6086–6091
Nishikawa H, Koyama S (2021) Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. https://doi.org/10.1136/jitc-2021-002591
Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B et al (2019) The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer 19:9–31
Wang Y, Ding Y, Guo N, Wang S (2019) MDSCs: key criminals of tumor pre-metastatic niche formation. Front Immunol 10:172
Li C, Jiang P, Wei S, Xu X, Wang J (2020) Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer 19:116
Yano H, Andrews LP, Workman CJ, Vignali DAA (2019) Intratumoral regulatory T cells: markers, subsets and their impact on anti-tumor immunity. Immunology 157:232–247
Kim JH, Kim BS, Lee SK (2020) Regulatory T Cells in tumor microenvironment and approach for anticancer immunotherapy. Immune Netw. https://doi.org/10.4110/in.2020.20.e4
deLeeuw RJ, Kost SE, Kakal JA, Nelson BH (2012) The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res 18:3022–3029
Paluskievicz CM, Cao X, Abdi R, Zheng P, Liu Y, Bromberg JS (2019) T regulatory cells and priming the suppressive tumor microenvironment. Front Immunol 10:2453
Sakaguchi S (2004) Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 22:531–562
Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I et al (2013) Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA 110:17945–17950
Villarreal DO, L’Huillier A, Armington S, Mottershead C, Filippova EV, Coder BD et al (2018) Targeting CCR8 induces protective antitumor immunity and enhances vaccine-induced responses in colon cancer. Cancer Res 78:5340–5348
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16:582–598
Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM et al (2020) A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 20:174–186
Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H et al (2018) Cancer-associated fibroblasts affect intratumoral CD8(+) and FoxP3(+) T cells Via IL6 in the tumor microenvironment. Clin Cancer Res 24:4820–4833
Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X et al (2016) Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell 30:243–256
Acknowledgements
We thank Dr. Jessica Tamanini from Insight Editing London for critical reading of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 2021YFA1201102, 82073231) and the Henan Province Scientific and Technological Research (Grant Nos. 222102310728).
Author information
Authors and Affiliations
Contributions
MW and ZHQ designed the project. MW and ZYQ conducted most of the experiments. YY and XXD performed animal experiments. JJW and XHY responsible for immunofluorescence staining. ZMJ, WQL performed some of the cell culture experiments. MW and ZYQ analyzed the data. MW and ZYQ wrote and edited the manuscript. All authors have read and agreed the contents of the manuscript and consent to its publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All animal experiments were approved by the Ethical Review Committee of the First Affiliated Hospital of Zhengzhou University under approval number 2020-KY-154.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, M., Qin, Z., Wan, J. et al. Tumor-derived exosomes drive pre-metastatic niche formation in lung via modulating CCL1+ fibroblast and CCR8+ Treg cell interactions. Cancer Immunol Immunother 71, 2717–2730 (2022). https://doi.org/10.1007/s00262-022-03196-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03196-3