Abstract
Purpose
Histopathologic prognostic factors of rectal cancer are closely associated with local recurrence and distant metastasis. We aim to investigate the feasibility of T2*WI in assessment of clinical prognostic factors of rectal cancer, and compare with DKI.
Methods
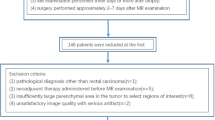
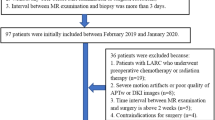
This retrospective study enrolled 50 out of 205 patients with rectal cancer according to the inclusion criteria. The following parameters were obtained: R2* from T2*WI, mean diffusivity (MDk), mean kurtosis (MK), and mean diffusivity (MDt) from DKI using tensor method. Above parameters were compared by Mann–Whitney U-test or students’ t test. Spearman correlations between different parameters and histopathological prognostic factors were determined. The diagnostic performances of R2* and DKI-derived parameters were analyzed by receiver operating characteristic curves (ROC), separately and jointly.
Results
There were positive correlations between R2* and multiple prognostic factors of rectal cancer such as T category, N category, tumor grade, CEA level, and LVI (P < 0.004). MDk and MDt showed negative correlations with almost all the histopathological prognostic factors except CRM and TIL involvement (P < 0.003). MK correlated positively with the prognostic factors except CA19-9 level and CRM involvement (P < 0.006). The AUC ranges were 0.724–0.950 for R2* and 0.755–0.913 for DKI-derived parameters for differentiation of prognostic factors. However, no significant differences of diagnostic performance were found between T2*WI, DKI, or the combined imaging methods in characterizing rectal cancer.
Conclusion
R2* and DKI-derived parameters were associated with different histopathological prognostic factors, and might act as noninvasive biomarkers for histopathological characterization of rectal cancer.





Similar content being viewed by others
References
Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365(9454):153-65. https://doi.org/https://doi.org/10.1016/S0140-6736(05)17706-X
Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. J Clin Pathol. 2008;61(5):561-9. https://doi.org/https://doi.org/10.1136/jcp.2007.054858
Sun H, Xu Y, Song A, Shi K, Wang W. Intravoxel Incoherent Motion MRI of Rectal Cancer: Correlation of Diffusion and Perfusion Characteristics With Prognostic Tumor Markers. AJR Am J Roentgenol. 2018;210(4):W139-W47. https://doi.org/https://doi.org/10.2214/AJR.17.18342
Lee JH, Kim SH, Jang HS, Chung HJ, Oh ST, Lee DS, et al. Preoperative elevation of carcinoembryonic antigen predicts poor tumor response and frequent distant recurrence for patients with rectal cancer who receive preoperative chemoradiotherapy and total mesorectal excision: a multi-institutional analysis in an Asian population. Int J Colorectal Dis. 2013;28(4):511-7. https://doi.org/https://doi.org/10.1007/s00384-012-1584-6
Shin YR, Kim KA, Im S, Hwang SS, Kim K. Prediction of KRAS Mutation in Rectal Cancer Using MRI. Anticancer Res. 2016;36(9):4799–804. https://doi.org/10.21873/anticanres.11039
Bostel T, Dreher C, Wollschlager D, Mayer A, Konig F, Bickelhaupt S, et al. Exploring MR regression patterns in rectal cancer during neoadjuvant radiochemotherapy with daily T2- and diffusion-weighted MRI. Radiat Oncol. 2020;15(1):171. https://doi.org/https://doi.org/10.1186/s13014-020-01613-4
van der Sande ME, Beets GL, Hupkens BJ, Breukink SO, Melenhorst J, Bakers FC, et al. Response assessment after (chemo)radiotherapy for rectal cancer: Why are we missing complete responses with MRI and endoscopy? Eur J Surg Oncol. 2019;45(6):1011-7. https://doi.org/https://doi.org/10.1016/j.ejso.2018.11.019
Cui Y, Cui X, Yang X, Zhuo Z, Du X, **n L, et al. Diffusion kurtosis imaging-derived histogram metrics for prediction of KRAS mutation in rectal adenocarcinoma: Preliminary findings. Journal of magnetic resonance imaging : JMRI. 2019;50(3):930-9. https://doi.org/https://doi.org/10.1002/jmri.26653
Granata V, Fusco R, Reginelli A, Delrio P, Selvaggi F, Grassi R, et al. Diffusion kurtosis imaging in patients with locally advanced rectal cancer: current status and future perspectives. J Int Med Res. 2019;47(6):2351-60. https://doi.org/https://doi.org/10.1177/0300060519827168
Sun Y, **ao Q, Hu F, Fu C, Jia H, Yan X, et al. Diffusion kurtosis imaging in the characterisation of rectal cancer: utilizing the most repeatable region-of-interest strategy for diffusion parameters on a 3T scanner. European radiology. 2018;28(12):5211-20. https://doi.org/https://doi.org/10.1007/s00330-018-5495-y
Yu J, Xu Q, Song JC, Li Y, Dai X, Huang DY, et al. The value of diffusion kurtosis magnetic resonance imaging for assessing treatment response of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. European radiology. 2017;27(5):1848-57. https://doi.org/https://doi.org/10.1007/s00330-016-4529-6
Zhang XY, Wang L, Zhu HT, Li ZW, Ye M, Li XT, et al. Predicting Rectal Cancer Response to Neoadjuvant Chemoradiotherapy Using Deep Learning of Diffusion Kurtosis MRI. Radiology. 2020;296(1):56-64. https://doi.org/https://doi.org/10.1148/radiol.2020190936
Cui Y, Yang X, Du X, Zhuo Z, **n L, Cheng X. Whole-tumour diffusion kurtosis MR imaging histogram analysis of rectal adenocarcinoma: Correlation with clinical pathologic prognostic factors. European radiology. 2018;28(4):1485-94. https://doi.org/https://doi.org/10.1007/s00330-017-5094-3
Wen Z, Chen Y, Yang X, Lu B, Liu Y, Shen B, et al. Application of magnetic resonance diffusion kurtosis imaging for distinguishing histopathologic subtypes and grades of rectal carcinoma. Cancer imaging : the official publication of the International Cancer Imaging Society. 2019;19(1):8. https://doi.org/https://doi.org/10.1186/s40644-019-0192-x
Zhu L, Pan Z, Ma Q, Yang W, Shi H, Fu C, et al. Diffusion Kurtosis Imaging Study of Rectal Adenocarcinoma Associated with Histopathologic Prognostic Factors: Preliminary Findings. Radiology. 2017;284(1):66-76. https://doi.org/https://doi.org/10.1148/radiol.2016160094
Rodrigues LM, Howe FA, Griffiths JR, Robinson SP. Tumor R2* is a prognostic indicator of acute radiotherapeutic response in rodent tumors. Journal of magnetic resonance imaging : JMRI. 2004;19(4):482-8. https://doi.org/https://doi.org/10.1002/jmri.20024
Vink EE, Boer A, Verloop WL, Spiering W, Voskuil M, Vonken E, et al. The effect of renal denervation on kidney oxygenation as determined by BOLD MRI in patients with hypertension. European radiology. 2015;25(7):1984-92. https://doi.org/https://doi.org/10.1007/s00330-014-3583-1
Li SP, Padhani AR, Makris A. Dynamic contrast-enhanced magnetic resonance imaging and blood oxygenation level-dependent magnetic resonance imaging for the assessment of changes in tumor biology with treatment. J Natl Cancer Inst Monogr. 2011;2011(43):103-7. https://doi.org/https://doi.org/10.1093/jncimonographs/lgr031
Zhang YD, Wu CJ, Wang Q, Zhang J, Wang XN, Liu XS, et al. Comparison of Utility of Histogram Apparent Diffusion Coefficient and R2* for Differentiation of Low-Grade From High-Grade Clear Cell Renal Cell Carcinoma. AJR Am J Roentgenol. 2015;205(2):W193-201. https://doi.org/https://doi.org/10.2214/AJR.14.13802
Wang Y, Shen Y, Hu X, Li Z, Feng C, Hu D, et al. Application of R2* and Apparent Diffusion Coefficient in Estimating Tumor Grade and T Category of Bladder Cancer. AJR Am J Roentgenol. 2020;214(2):383-9. https://doi.org/https://doi.org/10.2214/AJR.19.21668
Hallac RR, Ding Y, Yuan Q, McColl RW, Lea J, Sims RD, et al. Oxygenation in cervical cancer and normal uterine cervix assessed using blood oxygenation level-dependent (BOLD) MRI at 3T. NMR in biomedicine. 2012;25(12):1321-30. https://doi.org/https://doi.org/10.1002/nbm.2804
Peng Y, Luo Y, Hu X, Shen Y, Hu D, Li Z, et al. Quantitative T2*-Weighted Imaging and Reduced Field-of-View Diffusion-Weighted Imaging of Rectal Cancer: Correlation of R2* and Apparent Diffusion Coefficient With Histopathological Prognostic Factors. Frontiers in oncology. 2021;11:670156. https://doi.org/https://doi.org/10.3389/fonc.2021.670156
Tabesh A, Jensen JH, Ardekani BA, Helpern JA. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med. 2011;65(3):823-36. https://doi.org/https://doi.org/10.1002/mrm.22655
Liang J, Ma R, Chen H, Zhang D, Ye W, Shi C, et al. Detection of Hyperacute Reactions of Desacetylvinblastine Monohydrazide in a Xenograft Model Using Intravoxel Incoherent Motion DWI and R2* Map**. AJR Am J Roentgenol. 2019;212(4):717-26. https://doi.org/https://doi.org/10.2214/AJR.18.20517
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. https://doi.org/10.1245/s10434-010-0985-4
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-45
Liu M, Guo X, Wang S, ** M, Wang Y, Li J, et al. BOLD-MRI of breast invasive ductal carcinoma: correlation of R2* value and the expression of HIF-1alpha. European radiology. 2013;23(12):3221-7. https://doi.org/https://doi.org/10.1007/s00330-013-2937-4
Huang Y, Lin Y, Hu W, Ma C, Lin W, Wang Z, et al. Diffusion Kurtosis at 3.0T as an in vivo Imaging Marker for Breast Cancer Characterization: Correlation With Prognostic Factors. Journal of magnetic resonance imaging : JMRI. 2019;49(3):845–56. https://doi.org/10.1002/jmri.26249
Hectors SJ, Semaan S, Song C, Lewis S, Haines GK, Tewari A, et al. Advanced Diffusion-weighted Imaging Modeling for Prostate Cancer Characterization: Correlation with Quantitative Histopathologic Tumor Tissue Composition-A Hypothesis-generating Study. Radiology. 2018;286(3):918-28. https://doi.org/https://doi.org/10.1148/radiol.2017170904
Budjan J, Sauter EA, Zoellner FG, Lemke A, Wambsganss J, Schoenberg SO, et al. Diffusion kurtosis imaging of the liver at 3 Tesla: in vivo comparison to standard diffusion-weighted imaging. Acta Radiol. 2018;59(1):18-25. https://doi.org/https://doi.org/10.1177/0284185117706608
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432-40. https://doi.org/https://doi.org/10.1002/mrm.20508
Ianus A, Santiago I, Galzerano A, Montesinos P, Loucao N, Sanchez-Gonzalez J, et al. Higher-order diffusion MRI characterization of mesorectal lymph nodes in rectal cancer. Magn Reson Med. 2020;84(1):348-64. https://doi.org/https://doi.org/10.1002/mrm.28102
Rosenkrantz AB, Padhani AR, Chenevert TL, Koh DM, De Keyzer F, Taouli B, et al. Body diffusion kurtosis imaging: Basic principles, applications, and considerations for clinical practice. Journal of Magnetic Resonance Imaging : JMRI. 2015;42(5):1190-202. https://doi.org/https://doi.org/10.1002/jmri.24985
Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue characterization. NMR in biomedicine. 2010;23(7):836-48. https://doi.org/https://doi.org/10.1002/nbm.1506
Nogueira L, Brandao S, Matos E, Nunes RG, Loureiro J, Ramos I, et al. Application of the diffusion kurtosis model for the study of breast lesions. European radiology. 2014;24(6):1197-203. https://doi.org/https://doi.org/10.1007/s00330-014-3146-5
Jansen JF, Stambuk HE, Koutcher JA, Shukla-Dave A. Non-gaussian analysis of diffusion-weighted MR imaging in head and neck squamous cell carcinoma: A feasibility study. AJNR Am J Neuroradiol. 2010;31(4):741-8. https://doi.org/https://doi.org/10.3174/ajnr.A1919
Funding
This work was supported by the National Science Fund for Distinguished Young Scholars of China (No. 81925023) and the National Natural Science Foundation of China (No. 82071892).
Author information
Authors and Affiliations
Contributions
LCH are the guarantors of integrity of the entire study. HS, PY, LZY helped in the study concepts and design. HS and PY conducted the literature research. HS, PY, and WQS conducted the clinical studies. HS, PY, LB, and WQS conducted the experimental studies and data analysis. LB conducted the statistical analysis. HS and PY prepared and edited the manuscript. IK reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted retrospectively under the approval of the Ethics Committee of Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences. Written informed consent for participants was waived for this investigation according to the national legislation and the institutional requirements.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, S., Peng, Y., Wang, Q. et al. T2*-weighted imaging and diffusion kurtosis imaging (DKI) of rectal cancer: correlation with clinical histopathologic prognostic factors. Abdom Radiol 47, 517–529 (2022). https://doi.org/10.1007/s00261-021-03369-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03369-1