Abstract
Purpose
Advanced gastric cancer (AGC) and primary gastric lymphoma (PGL) are the two most common malignant tumors of the stomach. Conventional imaging examinations have difficulty distinguishing the two. This study explored the values of multiple parameters and visual assessment of 18F-fluorodeoxyglucose(18F-FDG) uptake heterogeneity of positron emission tomography/computed tomography(PET/CT) for differentiating between AGC and PGL.
Methods
This retrospective study included 70 AGC and 26 PGL patients, all of whom had undergone 18F-FDG PET/CT before treatment. We analyzed the differences between AGC and PGL in the distribution of metastatic lesions and multiple metabolic parameters, including the maximum standardized uptake value (SUVmax), SUVmax/maximal thickness(THKmax), metabolic tumor volume and total lesion glycolysis (TLG). In addition, 18F-FDG uptake heterogeneity was visually assessed using a visual scoring method and a method of measuring SUVmax differences (SUVmax-d).
Results
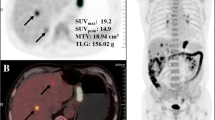
The most common metastasis of AGC patients were liver, bone, peritoneal and proximal lymph nodes; PGL patients had fewer peritoneal metastases and lymph node metastasis could appeared to be “skip metastasis.” The metabolic parameters—SUVmax, SUVmax/THKmax and TLG—were higher in patients who had PGL, especially in diffuse large B-cell lymphoma (DLBCL). In the visual assessment of 18F-FDG uptake heterogeneity, the measurements of SUVmax-d in PGL were significantly higher than in AGC. Receiver operating characteristics curve analysis suggested that SUVmax has the highest comprehensive diagnostic efficiency due to having the highest value of area under the curve and the highest accuracy (77.2%).
Conclusion
18F-FDG PET/CT had a high diagnostic efficiency for discrimination of AGC and PGL, especially between DLBCL and other pathological subtypes. Visual assessment used to evaluate 18F-FDG uptake heterogeneity could help to distinguish the two types of tumors. In addition, our innovative method of measuring the heterogeneity of 18F-FDG uptake—namely, SUVmax-d—could contribute to identification of the two tumor types and should have its significance clarified by future studies.




Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. (2018) Cancer statistics. CA Cancer J Clin 68: 7-30.
Kajitani T. (1981) The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg 11: 127-139.
Maconi G, Manes G, Porro GB. (2008) Role of symptoms in diagnosis and outcome of gastric cancer. World J Gastroenterol 14: 1149-1155.
Al-Akwaa AM, Siddiqui N, Al-Mofleh IA. (2004)Primary gastric lymphoma. World J Gastroenterol 10: 5-11.
Ghimire P, Wu GY, Zhu L. (2011) Primary gastrointestinal lymphoma. World J Gastroenterol 17: 697-707.
Juárez-Salcedo LM, Sokol L, Chavez JC, Dalia S. (2018)Primary Gastric Lymphoma, Epidemiology, Clinical Diagnosis, and Treatment. Cancer Control 25: 1-12.
Medina-Franco H, Germes SS, Maldonado CL. (2007)Prognostic factors in primary gastric lymphoma. Ann Surg Oncol 14: 2239-2245.
Wang YG, Zhao LY, Liu CQ, et al. (2016)Clinical characteristics and prognostic factors of primary gastric lymphoma: A retrospective study with 165 cases. Medicine (Baltimore) 95: e4250.
Fan WJ, Lu YC, Liu LZ, et al. (2008)Comparison of CT findings between gastric cancer and gastric lymphoma. Chin J Cancer 27: 539-543.
Chou CK, Chen LT, Sheu RS, et al. (1994)MRI manifestations of gastrointestinal lymphoma. Abdom Imaging 19: 495-500.
Cui X, Zhou T, Jiang D, et al. (2017)Clinical manifestations and endoscopic presentations of gastric lymphoma: a multicenter seven year retrospective survey. Rev Esp Enferm Dig 109: 566-571.
Okasha HH, Naguib M, El NM, et al. (2017)Role of endoscopic ultrasound and endoscopic-ultrasound-guided fine-needle aspiration in endoscopic biopsy negative gastrointestinal lesions. Endosc Ultrasound 6: 156-161.
Maekawa A, Kato M, Nakamura T, et al. (2018)Incidence of gastric adenocarcinoma among lesions diagnosed as low-grade adenoma/dysplasia on endoscopic biopsy: A multicenter, prospective, observational study. Dig Endosc 30: 228-235.
Ye Y, Tan S. (2018)Endoscopic ultrasound-guided fine-needle aspiration biopsy for diagnosis of gastric linitis plastica with negative malignant endoscopy biopsies. Oncol Lett 16: 4915-4920.
Kitajima K, Nakajo M, Kaida H, et al. (2017)Present and future roles of FDG-PET/CT imaging in the management of gastrointestinal cancer: an update. Nagoya J Med Sci 79: 527-543.
Findlay JM, Antonowicz S, Segaran A, et al. (2019)Routinely staging gastric cancer with 18F-FDG PET-CT detects additional metastases and predicts early recurrence and death after surgery. Eur Radiol 29: 2490-2498.
Yoh T, Seo S, Morino K, et al. (2019)Reappraisal of Prognostic Impact of Tumor SUVmax by 18F-FDG-PET/CT in Intrahepatic Cholangiocarcinoma. World J Surg 43: 1323-1331.
Qin C, Yang S, Sun X, **a X, Li C, Lan X. (2019)18F-FDG PET/CT for Prognostic Stratification of Patients With Extranodal Natural Killer/T-Cell Lymphoma. Clin Nucl Med 44: 201-208.
Li XF, Fu Q, Dong YW, et al. (2016)(18)F-fluorodeoxyglucose positron emission tomography/computed tomography comparison of gastric lymphoma and gastric carcinoma. World J Gastroenterol 22: 7787-96.
Fu L, Li H, Wang H, Xu B, Fan Y, Tian J. (2012)SUVmax/THKmax as a biomarker for distinguishing advanced gastric carcinoma from primary gastric lymphoma. PLoS One 7: e50914.
Watabe T, Tatsumi M, Watabe H, et al. (2012)Intratumoral heterogeneity of F-18 FDG uptake differentiates between gastrointestinal stromal tumors and abdominal malignant lymphomas on PET/CT. Ann Nucl Med 26: 222-227.
Tixier F, Hatt M, Valla C, et al. (2014)Visual versus quantitative assessment of intratumor 18F-FDG PET uptake heterogeneity: prognostic value in non-small cell lung cancer. J Nucl Med 55: 1235-1241.
Ye J, Ren Y, Wei Z, et al. (2018)External validation of a modified 8th AJCC TNM system for advanced gastric cancer: Long-term results in southern China. Surg Oncol 27: 146-153.
Miwa K, Inubushi M, Wagatsuma K, et al. (2014)FDG uptake heterogeneity evaluated by fractal analysis improves the differential diagnosis of pulmonary nodules. Eur J Radiol 83: 715-719.
Tomita M, Ayabe T, Tsuchiya K, Nakamura K. (2018)Fluorodeoxyglucose Positron Emission Tomography Can Provide Useful Information for Differentiating Thymic Epithelial Tumors. Thorac Cardiovasc Surg 66: 345-349.
Matias Riihimäki, Hemminki A, Sundquist K, et al. (2016)Metastatic spread in patients with gastric cancer[J]. Oncotarget 7:52307-52316.
Ichiyoshi Y, Toda T, Nagasaki S, et al. (1993)Surgical approaches in primary gastric lymphoma and carcinoma[J]. International surgery 78:103-106.
Maruyama K, Gunvén P, Okabayashi K, et al. (1989)Lymph node metastases of gastric cancer. General pattern in 1931 patients.[J]. Annals of Surgery 210:596-602.
Dong M, Liu J, Sun X, **ng L. (2017)Prognositc significance of SUVmax on pretreatment 18 F-FDG PET/CT in early-stage non-small cell lung cancer treated with stereotactic body radiotherapy: A meta-analysis. J Med Imaging Radiat Oncol 61: 652-659.
Stahl A, Ott K, Weber W, et al. (2003)FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings[J]. Eur J Nucl Med Mol Imaging 30:288-295.
Kim S K, Kang K W, Lee J S, et al. (2006)Assessment of lymph node metastases using18F-FDG PET in patients with advanced gastric cancer[J]. European Journal of Nuclear Medicine & Molecular Imaging 33:148-155.
Albano D, Bertoli M, Ferro P, et al. (2016)18F-FDG PET/CT in gastric MALT lymphoma: a bicentric experience[J]. European journal of nuclear medicine and molecular imaging 44:1-9.
MCF C, SVS G, Frings V, et al. (2019)Partial-volume correction in dynamic PET-CT: effect on tumor kinetic parameter estimation and validation of simplified metrics. EJNMMI Res 9: 12.
Shi S, Ji S, Qin Y, et al. (2015)Metabolic tumor burden is associated with major oncogenomic alterations and serum tumor markers in patients with resected pancreatic cancer. Cancer Lett 360: 227-233.
Morita T, Tatsumi M, Ishibashi M, et al. (2017)Assessment of Mediastinal Tumors Using SUVmax and Volumetric Parameters on FDG-PET/CT. Asia Ocean J Nucl Med Biol 5: 22-29.
Husby JA, Reitan BC, Biermann M, et al. (2015)Metabolic Tumor Volume on 18F-FDG PET/CT Improves Preoperative Identification of High-Risk Endometrial Carcinoma Patients. J Nucl Med 56: 1191-1198.
Nakajo M, **guji M, Nakabeppu Y, et al. (2017)Texture analysis of 18F-FDG PET/CT to predict tumour response and prognosis of patients with esophageal cancer treated by chemoradiotherapy. Eur J Nucl Med Mol Imaging 44: 206-214.
Yoo SH, Kang SY, Cheon GJ, Oh DY, Bang YJ. (2019)Predictive Role of Temporal Changes in Intratumoral Metabolic Heterogeneity during Palliative Chemotherapy in Patients with Advanced Pancreatic Cancer: A Prospective Cohort Study. J Nucl Med 119:226407.
Liu F, Wang Z, Zhou X, et al.(2018)Genetic heterogeneity and mutational signature in Chinese Epstein-Barr virus-positive diffuse large B-cell lymphoma. PLoS One 13:e0201546.
Chapuy B, Cheng H, Watahiki A, et al. (2016)Diffuse large B-cell lymphoma patient-derived xenograft models capture the molecular and biological heterogeneity of the disease. Blood 127:2203-2213.
Kahle XU, Hovingh M, Noordzij W, et al. (2019)Tumour necrosis as assessed with 18F-FDG PET is a potential prognostic marker in diffuse large B cell lymphoma independent of MYC rearrangements. Eur Radiol 29:6018-6028.
Zhang J, **e Y, Wu Q, **a Y. (2019)Medical image classification using synergic deep learning. Med Image Anal 54:10-19.
Funding
This study was funded by the Education Department of Gansu Province (2018B-008), and Lanzhou University Second Hospital Cuiying Science and Technology Innovation Project (CY2017-BJ12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
This retrospective study was approved by our institutional review board. Informed written consent was waived given the retrospective nature of the work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, Y., Liu, J., Wang, L. et al. Multiple metabolic parameters and visual assessment of 18F-FDG uptake heterogeneity of PET/CT in advanced gastric cancer and primary gastric lymphoma. Abdom Radiol 45, 3569–3580 (2020). https://doi.org/10.1007/s00261-020-02503-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02503-9