Abstract
Background
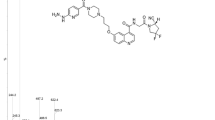
The aim of the present study was to develop a novel 64Cu-labeled cyclic peptide ([64Cu]Cu-FAP-NOX) that targets fibroblast activation protein (FAP) and may offer advantages in terms of image contrast, imaging time window, and low uptake in normal tissues.
Methods
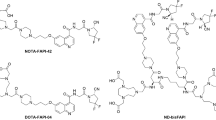
The novel cyclic peptide featuring with a N-oxalyl modified tail was constructed and conjugated to NOTA for 64Cu labeling. Biochemical and cellular assays were performed with A549.hFAP cells. The performance of [64Cu]Cu-FAP-NOX was compared to that of two established tracers ([64Cu]Cu-FAPI-04 and [68Ga]Ga-FAP-2286) and three different NOTA-conjugates in HEK-293T.hFAP xenograft mice using micro-PET imaging. Ex vivo biodistribution studies were performed to confirm the FAP specificity and to validate the PET data. Furthermore, a first-in-human study of this novel tracer was conducted on one patient with lung cancer.
Results
Compared to [64Cu]Cu-FAPI-04, [64Cu]Cu-FAP-NOX demonstrated faster and higher rates of cellular uptake and internalization in A549.hFAP cells, but lower rates of cellular efflux. All six radiotracers were rapidly taken up by the tumor within the first 4 h post-injection. However, [64Cu]Cu-FAP-NOX had more intense tumor accumulation and slower washout from the target. The ratios of the tumor to normal tissue (including kidneys and muscles) increased significantly over time, with [64Cu]Cu-FAP-NOX reaching the highest ratio among all tracers. In the patient, [64Cu]Cu-FAP-NOX PET showed a comparable result to FDG PET in the primary malignant lesion while exhibiting higher uptake in pleural metastases, consistent with elevated FAP expression as confirmed by immunohistochemistry.
Conclusion
[64Cu]Cu-FAP-NOX is a promising FAP-targeted tracer with a highly flexible imaging time window, as evidenced by preclinical evaluation encompassing biodistribution and micro-PET studies, along with a successful patient application. Furthermore, [64Cu]Cu-FAP-NOX showed enhanced image contrast and favorable pharmacokinetic properties for FAP PET imaging, warranting translation into large cohort studies.







Similar content being viewed by others
References
Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39(3):783–803.
Peltier A, Seban RD, Buvat I, Bidard FC, Mechta-Grigoriou F. Fibroblast heterogeneity in solid tumors: from single cell analysis to whole-body imaging. Semin Cancer Biol. 2022;86(3):262–72.
Ora M, Soni N, Nazar AH, Dixit M, Singh R, Puri S, Graham MM, Gambhir S. Fibroblast activation protein inhibitor-based radionuclide therapies. Current status and future directions. J Nucl Med. 2023;64(7):1001–8.
Altmann A, Haberkorn U, Siveke J. The latest developments in imaging of fibroblast activation protein. J Nucl Med. 2021;62(2):160–7.
Šimková A, Bušek P, Šedo A, Konvalinka J. Molecular recognition of fibroblast activation protein for diagnostic and therapeutic applications. Biochim Biophys Acta Proteins Proteom. 2020;1868(7):140409.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, Adeberg S, Rathke H, Röhrich M, Winter H, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60(6):801–5.
Hu K, Wang L, Wu H, Huang S, Tian Y, Wang Q, **ao C, Han Y, Tang G. [18F]FAPI-42 PET imaging in cancer patients: optimal acquisition time, biodistribution, and comparison with [68Ga]Ga-FAPI-04. Eur J Nucl Med Mol Imaging. 2022;49(8):2833–43.
Shi X, **ng H, Yang X, Li F, Yao S, Zhang H, Zhao H, Hacker M, Huo L, Li X. Fibroblast imaging of hepatic carcinoma with 68Ga-FAPI-04 PET/CT: a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging. 2021;48(1):196–203.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, Jäger D, Mier W, Haberkorn U. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59(9):1415–22.
Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, Kratochwil C, Debus J, Marmé F, Jäger D, Mier W, et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J Nucl Med. 2019;60(10):1421–9.
Pang Y, Zhao L, Chen H. 68Ga-FAPI outperforms 18F-FDG PET/CT in identifying bone metastasis and peritoneal carcinomatosis in a patient with metastatic breast cancer. Clin Nucl Med. 2020;45(11):913–5.
Zboralski D, Hoehne A, Bredenbeck A, Schumann A, Nguyen M, Schneider E, Ungewiss J, Paschke M, Haase C, von Hacht JL, et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur J Nucl Med Mol Imaging. 2022;49(11):3651–67.
Baum RP, Schuchardt C, Singh A, Chantadisai M, Robiller FC, Zhang J, Mueller D, Eismant A, Almaguel F, Zboralski D, et al. Feasibility, biodistribution, and preliminary dosimetry in peptide-targeted radionuclide therapy of diverse adenocarcinomas using 177Lu-FAP-2286: first-in-humans results. J Nucl Med. 2022;63(3):415–23.
Zboralski D, Osterkamp F, Christensen E, Bredenbeck A, Schumann A, Hoehne A, Schneider E, Paschke M, Ungewiss J, Haase C, et al. Fibroblast activation protein targeted radiotherapy induces an immunogenic tumor microenvironment and enhances the efficacy of PD-1 immune checkpoint inhibition. Eur J Nucl Med Mol Imaging. 2023;50(9):2621–35.
Kline B, Yadav S, Seo Y, Ippisch RC, Castillo J, Aggarwal RR, Kelley RK, Behr SC, Flavell RR, Lawhn-Heath C, et al. Ga-FAP-2286 PET of solid tumors: Biodistribution, Dosimetry, and comparison with 18F-FDG. J Nucl Med. 2024;68(6):938–43.
Pang Y, Zhao L, Meng T, Xu W, Lin Q, Wu H, Zhang J, Chen X, Sun L, Chen H. PET imaging of fibroblast activation protein in various types of cancer using 68Ga-FAP-2286: comparison with 18F-FDG and 68Ga-FAPI-46 in a single-center, prospective study. J Nucl Med. 2023;64(3):386–94.
Banihashemian SS, Divband G, Pirayesh E, Nikkholgh B, Amini H, Shahrnoy AA, Nami R, Akbari ME. [68Ga]Ga-FAP-2286, a novel promising theragnostic approach for PET/CT imaging in patients with various type of metastatic cancers. Eur J Nucl Med Mol Imaging. 2024;51(7):1981–8.
Nayak TK, Brechbiel MW. Radioimmunoimaging with longer-lived positron-emitting radionuclides: potentials and challenges. Bioconjug Chem. 2009;20(5):825–41.
Chhabra A, Thakur ML. Theragnostic radionuclide pairs for prostate cancer management: 64Cu/67Cu, can be a budding hot duo. Biomedicines. 2022;10(11):2787.
Bailey DL, Willowson KP, Harris M, Biggin C, Aslani A, Lengkeek NA, Stoner J, Eslick ME, Marquis H, Parker M, et al. Cu treatment planning and 67Cu therapy with radiolabeled [64Cu/67Cu]MeCOSar-Octreotate in subjects with unresectable multifocal meningioma: initial results for human imaging, safety, biodistribution, and radiation dosimetry. J Nucl Med. 2023;64(5):704–10.
Krasnovskaya OO, Abramchuck D, Erofeev A, Gorelkin P, Kuznetsov A, Shemukhin A, Beloglazkina EK. Recent advances in 64Cu/67Cu-based radiopharmaceuticals. Int J Mol Sci. 2023;24(11):9154.
Maheshwari V, Dearling JLJ, Treves ST, Packard AB. Measurement of the rate of copper(II) exchange for 64Cu complexes of bifunctional chelators. Inorg Chim Acta. 2012;393:318–23.
Liu T, Liu C, Zhang Z, Zhang N, Guo X, **a L, Jiang J, **e Q, Yan K, Rowe SP, et al. 64Cu-PSMA-BCH: a new radiotracer for delayed PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48(13):4508–16.
Liu L, Zhong J, Zhang Z, Ye X, Wang X, Liu S, Zhang Z. Preclinical study and first-in-human imaging of [18F]FAP-2286, and comparison with 2-[18F]FDG PET/CT in various cancer patients. Eur J Nucl Med Mol Imaging. 2024;51(7):2012–22.
Jiang H, Chen W, Wang J, Zhang R. Selective N-terminal modification of peptides and proteins: recent progresses and applications. Chin Chem Lett. 2022;33(1):80–8.
Sui X, Tan H, Yu H, **ao J, Qi C, Cao Y, Chen S, Zhang Y, Hu P, Shi H. Exploration of the total-body PET/CT reconstruction protocol with ultra-low 18F-FDG activity over a wide range of patient body mass indices. EJNMMI Phys. 2022;9(1):17.
Xu L, Cui C, Li R, Yang R, Liu R, Meng Q, Wang F. Phantom and clinical evaluation of the effect of a new bayesian penalized likelihood reconstruction algorithm (HYPER iterative) on 68Ga-DOTA-NOC PET/CT image quality. EJNMMI Res. 2022;12(1):73.
Delpassand ES, Ranganathan D, Wagh N, Shafie A, Gaber A, Abbasi A, Kjaer A, Tworowska I, Núñez R. 64Cu-DOTATATE PET/CT for imaging patients with known or suspected somatostatin receptor-positive neuroendocrine tumors: results of the first U.S. prospective, reader-masked clinical trial. J Nucl Med. 2020;61(6):890–6.
Pfeifer A, Knigge U, Mortensen J, Oturai P, Berthelsen AK, Loft A, Binderup T, Rasmussen P, Elema D, Klausen TL, Holm S, et al. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: first-in-humans study. J Nucl Med. 2012;53(8):1207–15.
Lee MF, Poh CL. Strategies to improve the physicochemical properties of peptide-based drugs. Pharm Res. 2023;40(3):617–32.
Keinänen O, Fung K, Brennan JM, Zia N, Harris M, van Dam E, Biggin C, Hedt A, Stoner J, Donnelly PS, et al. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc Natl Acad Sci U S A. 2020;117(45):28316–27.
Hicks RJ, Jackson P, Kong G, Ware RE, Hofman MS, Pattison DA, Akhurst TA, Drummond E, Roselt P, Callahan J, et al. Cu-SARTATE PET imaging of patients with neuroendocrine tumors demonstrates high tumor uptake and retention, potentially allowing prospective dosimetry for peptide receptor radionuclide therapy. J Nucl Med. 2019;64(6):777–85.
Funding
This research was supported in part by the National Natural Science Foundation of China (No. 82001879), the Applied and Basic Research Foundation of Guangdong Province (No. 2020A1515110159), the Science and Technology Project of Guangzhou City (No. 202102010354, No. 202201020558 and No. 2024A03J1080).
Author information
Authors and Affiliations
Contributions
Shaoyu Liu and **nlu Wang conceived and designed this research. Jiawei Zhong, Ziqi Zhang, Qingsong Yan, and Ruiyue Zhao were responsible for most of the experiments, data collection, and analysis. The first draft of the manuscript was written by Shaoyu Liu, Jiawei Zhong and Ziqi Zhang. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
No other potential conflict of interest relevant to this article was reported.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Zhong, J., Zhang, Z. et al. [64Cu]Cu-FAP-NOX, a N-oxalyl modified cyclic peptide for FAP PET imaging with a flexible imaging time window. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06807-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06807-6