Abstract
Purpose
Exploring synaptic density changes during brain growth is crucial to understanding brain development. Previous studies in nonhuman primates report a rapid increase in synapse number between the late gestational period and the early neonatal period, such that synaptic density approaches adult levels by birth. Prenatal synaptic development may have an enduring impact on postnatal brain development, but precisely how synaptic density changes in utero are unknown because current methods to quantify synaptic density are invasive and require post-mortem brain tissue.
Methods
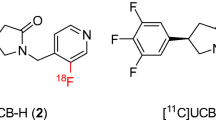
We used synaptic vesicle glycoprotein 2A (SV2A) positron emission tomography (PET) radioligands [11C]UCB-J and [18F]Syn-VesT-1 to conduct the first assessment of synaptic density in the develo** fetal brain in gravid rhesus monkeys. Eight pregnant monkeys were scanned twice during the third trimester at two imaging sites. Fetal post-mortem samples were collected near term in a subset of subjects to quantify SV2A density by Western blot.
Results
Image-derived fetal brain SV2A measures increased during the third trimester. SV2A concentrations were greater in subcortical regions than in cortical regions at both gestational ages. Near term, SV2A density was higher in primary motor and visual areas than respective associative regions. Post-mortem quantification of SV2A density was significantly correlated with regional SV2A PET measures.
Conclusion
While further study is needed to determine the exact relationship of SV2A and synaptic density, the imaging paradigm developed in the current study allows for the effective in vivo study of SV2A development in the fetal brain.






Similar content being viewed by others
Availability of data and material
The raw data and datasets used and/or analyzed in the current study will be made available upon reasonable request to the corresponding and participating authors as deemed appropriate, and in line with the data sharing policy.
Code of Availability
The code used in the current study will be made available upon reasonable request to the corresponding and participating authors as deemed appropriate, and in line with the data sharing policy.
References
Huttenlocher PR. Synaptic density in human frontal cortex — developmental changes and effects of aging. Brain Res. 1979;163:195–205. https://doi.org/10.1016/0006-8993(79)90349-4.
Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. https://doi.org/10.1002/(sici)1096-9861(19971020)387:2%3c167::aid-cne1%3e3.0.co;2-z.
Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex–evidence for synapse elimination during normal development. Neurosci Lett. 1982;33:247–52. https://doi.org/10.1016/0304-3940(82)90379-2.
Bourgeois JP, Jastreboff PJ, Rakic P. Synaptogenesis in visual cortex of normal and preterm monkeys: evidence for intrinsic regulation of synaptic overproduction. Proc Natl Acad Sci U S A. 1989;86:4297–301. https://doi.org/10.1073/pnas.86.11.4297.
Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–20.
Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–5. https://doi.org/10.1126/science.3952506.
Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res. 1989;50:11–32. https://doi.org/10.1016/0165-3806(89)90124-7.
Zecevic N, Rakic P. Synaptogenesis in monkey somatosensory cortex. Cereb Cortex. 1991;1:510–23. https://doi.org/10.1093/cercor/1.6.510.
Murray RM, O’Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18:319–32. https://doi.org/10.1093/schbul/18.2.319.
Volk DW, Edelson JR, Lewis DA. Altered expression of developmental regulators of parvalbumin and somatostatin neurons in the prefrontal cortex in schizophrenia. Schizophr Res. 2016;177:3–9. https://doi.org/10.1016/j.schres.2016.03.001.
Volk DW, Lewis DA. Early developmental disturbances of cortical inhibitory neurons: contribution to cognitive deficits in schizophrenia. Schizophr Bull. 2014;40:952–7. https://doi.org/10.1093/schbul/sbu111.
Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. https://doi.org/10.1001/archpsyc.1987.01800190080012.
Bajjalieh SM, Peterson K, Shinghal R, Scheller RH. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 1992;257:1271–3. https://doi.org/10.1126/science.1519064.
Nabulsi NB, Mercier J, Holden D, Carre S, Najafzadeh S, Vandergeten MC, et al. Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med. 2016;57:777–84. https://doi.org/10.2967/jnumed.115.168179.
Li S, Cai Z, Wu X, Holden D, Pracitto R, Kapinos M, et al. Synthesis and in vivo evaluation of a novel PET radiotracer for imaging of synaptic vesicle glycoprotein 2A (SV2A) in nonhuman primates. ACS Chem Neurosci. 2019;10:1544–54. https://doi.org/10.1021/acschemneuro.8b00526.
Chen MK, Mecca AP, Naganawa M, Finnema SJ, Toyonaga T, Lin SF, et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol. 2018;75:1215–24. https://doi.org/10.1001/jamaneurol.2018.1836.
Mecca AP, Chen MK, O’Dell RS, Naganawa M, Toyonaga T, Godek TA, et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 2020;16:974–82. https://doi.org/10.1002/alz.12097.
Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019;10:1529. https://doi.org/10.1038/s41467-019-09562-7.
Finnema SJ, Toyonaga T, Detyniecki K, Chen MK, Dias M, Wang Q, et al. Reduced synaptic vesicle protein 2A binding in temporal lobe epilepsy: A [(11) C]UCB-J positron emission tomography study. Epilepsia. 2020;61:2183–93. https://doi.org/10.1111/epi.16653.
Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra96. https://doi.org/10.1126/scitranslmed.aaf6667.
Tarantal AF (2005) Ultrasound Imaging in Rhesus (Macaca mulatta) and Long-Tailed (Macaca fascicularis) Macaques. Reproductive and Research Applications. In: The Laboratory Primate, Ch. 20. Elsevier Academic Press, Cambridge, pp 317–315
Berg E, Gill H, Marik J, Ogasawara A, Williams S, van Dongen G, et al. Total-body PET and highly stable chelators together enable meaningful (89)Zr-antibody PET studies up to 30 days after injection. J Nucl Med. 2020;61:453–60. https://doi.org/10.2967/jnumed.119.230961.
Berg E, Zhang X, Bec J, Judenhofer MS, Patel B, Peng Q, et al. Development and evaluation of mini-EXPLORER: a long axial field-of-view PET scanner for nonhuman primate imaging. J Nucl Med. 2018;59:993–8. https://doi.org/10.2967/jnumed.117.200519.
Young JT, Shi Y, Niethammer M, Grauer M, Coe CL, Lubach GR, et al. The UNC-Wisconsin Rhesus Macaque Neurodevelopment Database: a structural MRI and DTI database of early postnatal development. Front Neurosci. 2017;11:29. https://doi.org/10.3389/fnins.2017.00029.
Reveley C, Gruslys A, Ye FQ, Glen D, Samaha J, Russ BE, et al. Three-dimensional digital template atlas of the Macaque Brain. Cereb Cortex. 2017;27:4463–77. https://doi.org/10.1093/cercor/bhw248.
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40. https://doi.org/10.1097/00004647-199609000-00008.
Bartlett RM, Dejesus OT, Barnhart TE, Nickles RJ, Christian BT, Graner JL, et al. Fetal dopamine receptor characteristics assessed in utero. J Cereb Blood Flow Metab. 2010;30:1437–40. https://doi.org/10.1038/jcbfm.2010.77.
Tarantal AF, Hartigan-O’Connor DJ, Penna E, Kreutz A, Martinez ML, Noctor SC. Fetal Rhesus Monkey first trimester Zika virus infection impacts cortical development in the second and third trimesters. Cereb Cortex. 2021;31:2309–21. https://doi.org/10.1093/cercor/bhaa336.
Lancaster F, Delaney C, Samorajski T. Synaptic density of caudate-putamen and visual cortex following exposure to ethanol in utero. Int J Dev Neurosci. 1989;7:581–9. https://doi.org/10.1016/0736-5748(89)90017-8.
Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science. 1986;231:840–3. https://doi.org/10.1126/science.3945811.
Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–97. https://doi.org/10.1002/ana.410220408.
Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. https://doi.org/10.1002/cne.901450105.
McLeod F, Marzo A, Podpolny M, Galli S, Salinas P. Evaluation of synapse density in hippocampal rodent brain slices. J Vis Exp. 2017. https://doi.org/10.3791/56153.
Li M, Cui Z, Niu Y, Liu B, Fan W, Yu D, et al. Synaptogenesis in the develo** mouse visual cortex. Brain Res Bull. 2010;81:107–13. https://doi.org/10.1016/j.brainresbull.2009.08.028.
Crevecoeur J, Foerch P, Doupagne M, Thielen C, Vandenplas C, Moonen G, et al. Expression of SV2 isoforms during rodent brain development. BMC Neurosci. 2013;14:87. https://doi.org/10.1186/1471-2202-14-87.
Vanoye-Carlo A, Gomez-Lira G. Differential expression of SV2A in hippocampal glutamatergic and GABAergic terminals during postnatal development. Brain Res. 2019;1715:73–83. https://doi.org/10.1016/j.brainres.2019.03.021.
Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. https://doi.org/10.1016/s0306-4522(01)00171-3.
Nakamura H, Kobayashi S, Ohashi Y, Ando S. Age-changes of brain synapses and synaptic plasticity in response to an enriched environment. J Neurosci Res. 1999;56:307–15. https://doi.org/10.1002/(SICI)1097-4547(19990501)56:3%3c307::AID-JNR10%3e3.0.CO;2-3.
Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat II Quantitative analysis. J Neurocytol. 1983;12:697–712. https://doi.org/10.1007/BF01181531.
Mutch SA, Kensel-Hammes P, Gadd JC, Fujimoto BS, Allen RW, Schiro PG, et al. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci. 2011;31:1461–70. https://doi.org/10.1523/JNEUROSCI.3805-10.2011.
Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–46. https://doi.org/10.1016/j.cell.2006.10.030.
Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci U S A. 1999;96:15268–73. https://doi.org/10.1073/pnas.96.26.15268.
Hoffman EJ, Huang SC, Phelps ME. Quantitation in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr. 1979;3:299–308. https://doi.org/10.1097/00004728-197906000-00001.
Rossano S, Toyonaga T, Finnema SJ, Naganawa M, Lu Y, Nabulsi N, et al. Assessment of a white matter reference region for (11)C-UCB-J PET quantification. J Cereb Blood Flow Metab. 2020;40:1890–901. https://doi.org/10.1177/0271678X19879230.
Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–68. https://doi.org/10.1038/npp.2009.115.
Carson RE, Naganawa M, Matuskey D, Mecca A, Pittman B, Toyonaga T, et al. Age and sex effects on synaptic density in healthy humans as assessed with SV2A PET. Journal of Nuclear Medicine. 2018;59:541–541.
Michiels L, Delva A, van Aalst J, Ceccarini J, Vandenberghe W, Vandenbulcke M, et al. Synaptic density in healthy human aging is not influenced by age or sex: a (11)C-UCB-J PET study. Neuroimage. 2021;232: 117877. https://doi.org/10.1016/j.neuroimage.2021.117877.
Benveniste H, Fowler JS, Rooney WD, Moller DH, Backus WW, Warner DA, et al. Maternal-fetal in vivo imaging: a combined PET and MRI study. J Nucl Med. 2003;44:1522–30.
Benveniste H, Fowler JS, Rooney WD, Scharf BA, Backus WW, Izrailtyan I, et al. Cocaine is pharmacologically active in the nonhuman primate fetal brain. Proc Natl Acad Sci U S A. 2010;107:1582–7. https://doi.org/10.1073/pnas.0909585107.
Smart K, Liu H, Matuskey D, Chen MK, Torres K, Nabulsi N, et al. Binding of the synaptic vesicle radiotracer [(11)C]UCB-J is unchanged during functional brain activation using a visual stimulation task. J Cereb Blood Flow Metab. 2021;41:1067–79. https://doi.org/10.1177/0271678X20946198.
Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–98. https://doi.org/10.1016/s0306-4530(01)00050-6.
Acknowledgements
The authors acknowledge the nonhuman primate team at the Yale PET Center, including Daniel Holden, Cynthia Santaniello, Courtney Chabina, Kristin Koehl-Carabetta, and Amanda Harsche, for their assistance in completing the PET studies. The authors also acknowledge Dr. Alvaro Duque and the Rakic Breeding Colony for their assistance with NHP breeding at Yale and the staff at the California National Primate Research Center.
Funding
These studies were supported by National Institutes of Health (NIH) grants (no. MH120615 [SG], and no. U42-OD027094 [AT]), and the California National Primate Research Center base operating grant (no. OD011107). In vivo imaging was performed with instrumentation funded by the NIH (S10 grants RR029245, OD016261, and RR025063), and supported through the Primate Center Multimodal Imaging Core.
Author information
Authors and Affiliations
Contributions
SR collected and analyzed PET data, completed Western blotting and analysis, and prepared manuscript; TT supervised Western blotting and analysis, and manuscript preparation; EB collected and reconstructed PET data; IL analyzed PET data; KF performed PET imaging and data collection; NN, JR, SL, YY, ZF, and HH developed, synthesized, and QC of PET tracers; DK synthesized PET tracers; HB contributed to study design; AFT identified and assigned animals, performed all monitoring and imaging including ultrasound and PET/CT, data collection, performed all fetal tissue collections, contributed to study design, and manuscript preparation; SG analyzed PET data, contributed to study design, and manuscript preparation; REC contributed to study design, performed PET experiments, data analysis, and manuscript preparation.
Corresponding author
Ethics declarations
Ethics approval
All procedures were carried out in strict accordance to the guidelines set forth in the Animal Welfare Act and the Guide for the Care and Use of Laboratory animals. All animal procedures were approved prior to implementation by Yale University’s Institutional Animal Care and Use Committee (IACUC) and by the IACUC at the University of California, Davis.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rossano, S., Toyonaga, T., Berg, E. et al. Imaging the fetal nonhuman primate brain with SV2A positron emission tomography (PET). Eur J Nucl Med Mol Imaging 49, 3679–3691 (2022). https://doi.org/10.1007/s00259-022-05825-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05825-6