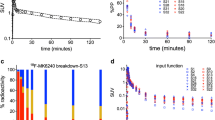
Abstract
Purpose
Recent studies have shown that standard compartmental models using plasma input or the cerebellum reference tissue input are generally not reliable for quantifying tau burden in dynamic 18F-flortaucipir PET studies of Alzheimer disease. So far, the optimal reference region for estimating 18F-flortaucipir delivery and specific tau binding has yet to be determined. The objective of the study is to improve 18F-flortaucipir brain tau PET quantification using a spatially constrained kinetic model with dual reference tissues.
Methods
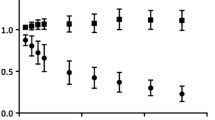
Participants were classified as either cognitively normal (CN) or cognitively impaired (CI) based on clinical assessment. T1-weighted structural MRI and 105-min dynamic 18F-flortaucipir PET scans were acquired for each participant. Using both a simplified reference tissue model (SRTM2) and Logan plot with either cerebellum gray matter or centrum semiovale (CS) white matter as the reference tissue, we estimated distribution volume ratios (DVRs) and the relative transport rate constant R1 for region of interest-based (ROI) and voxelwise-based analyses. Conventional linear regression (LR) and LR with spatially constrained (LRSC) parametric imaging algorithms were then evaluated. Noise-induced bias in the parametric images was compared to estimates from ROI time activity curve-based kinetic modeling. We finally evaluated standardized uptake value ratios at early phase (SUVREP, 0.7–2.9 min) and late phase (SUVRLP, 80–105 min) to approximate R1 and DVR, respectively.
Results
The percent coefficients of variation of R1 and DVR estimates from SRTM2 with spatially constrained modeling were comparable to those from the Logan plot and SUVRs. The SRTM2 using CS reference tissue with LRSC reduced noise-induced underestimation in the LR generated DVR images to negligible levels (< 1%). Inconsistent overestimation of DVR in the SUVRLP only occurred using the cerebellum reference tissue-based measurements. The CS reference tissue-based DVR and SUVRLP, and cerebellum-based SUVREP and R1 provided higher Cohen’s effect size d to detect increased tau deposition and reduced relative tracer transport rate in CI individuals.
Conclusion
Using a spatially constrained kinetic model with dual reference tissues significantly improved quantification of relative perfusion and tau binding. Cerebellum and CS are the suggested reference tissues to estimate R1 and DVR, respectively, for dynamic 18F-flortaucipir PET studies. Cerebellum-based SUVREP and CS-based SUVRLP may be used to simplify 18F-flortaucipir PET study.






Similar content being viewed by others
References
Kumar A, Singh A. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67:195–203.
Long JM, Holtzman DM. Alzheimer disease: An update on pathobiology and treatment strategies. Cell. 2019. https://doi.org/10.1016/j.cell.2019.09.001.
Gordon BA, McCullough A, Mishra S, Blazey TM, Su Y, Christensen J, et al. Cross-sectional and longitudinal atrophy is preferentially associated with tau rather than amyloid β positron emission tomography pathology. Alzheimer's Dement: Diagn, Assess Dis Monitor. 2018;10:245–52.
Giacobini E, Gold G. Alzheimer disease therapy—moving from amyloid-β to tau. Nat Rev Neurol. 2013;9:677.
Brion J-P. Neurofibrillary tangles and Alzheimer’s disease. Eur Neurol. 1998;40:130–40.
Graham WV, Bonito-Oliva A, Sakmar TP. Update on Alzheimer’s disease therapy and prevention strategies. Annu Rev Med. 2017;68:413–30.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Leuzy A, Chiotis K, Lemoine L, Gillberg P-G, Almkvist O, Rodriguez-Vieitez E, et al. Tau PET imaging in neurodegenerative tauopathies—still a challenge. Mol Psychiatry. 2019;1.
Hahn A, Schain M, Erlandsson M, Sjölin P, James GM, Strandberg OT, et al. Modeling strategies for quantification of in vivo 18F-AV-1451 binding in patients with tau pathology. J Nucl Med. 2017;58:623–31.
Barret O, Alagille D, Sanabria S, Comley RA, Weimer RM, Borroni E, et al. Kinetic modeling of the tau PET tracer 18F-AV-1451 in human healthy volunteers and Alzheimer disease subjects. J Nucl Med. 2017;58:1124–31.
Baker SL, Lockhart SN, Price JC, He M, Huesman RH, Schonhaut D, et al. Reference tissue–based kinetic evaluation of 18F-AV-1451 for tau imaging. J Nucl Med. 2017;58:332–8.
Shokouhi S, Mckay JW, Baker SL, Kang H, Brill AB, Gwirtsman HE, et al. Reference tissue normalization in longitudinal 18 F-florbetapir positron emission tomography of late mild cognitive impairment. Alzheimers Res Ther. 2016;8:2.
Golla SS, Timmers T, Ossenkoppele R, Groot C, Verfaillie S, Scheltens P, et al. Quantification of tau load using [18 F] AV1451 PET. Mol Imaging Biol. 2017;19:963–71.
Wooten DW, Guehl NJ, Verwer EE, Shoup TM, Yokell DL, Zubcevik N, et al. Pharmacokinetic evaluation of the tau PET radiotracer 18F-T807 (18F-AV-1451) in human subjects. J Nucl Med. 2017;58:484–91.
Martin-Facklam M, Pizzagalli F, Zhou Y, Ostrowitzki S, Raymont V, Brašić JR, et al. Glycine transporter type 1 occupancy by bitopertin: a positron emission tomography study in healthy volunteers. Neuropsychopharmacology. 2013;38:504–12.
Zhou Y, Resnick SM, Ye W, Fan H, Holt DP, Klunk WE, et al. Using a reference tissue model with spatial constraint to quantify [11C] Pittsburgh compound B PET for early diagnosis of Alzheimer’s disease. Neuroimage. 2007;36:298–312.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9.
Petrou M, Frey KA, Kilbourn MR, Scott PJ, Raffel DM, Bohnen NI, et al. In vivo imaging of human cholinergic nerve terminals with (−)-5-18F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med. 2014;55:396–404.
Koeppe R, Holthoff V, Frey K, Kilbourn M, Kuhl D. Compartmental analysis of [11C] flumazenil kinetics for the estimation of ligand transport rate and receptor distribution using positron emission tomography. J Cereb Blood Flow Metab. 1991;11:735–44.
Koeppe RA, Frey KA, Kume A, Albin R, Kilbourn MR, Kuhl DE. Equilibrium versus compartmental analysis for assessment of the vesicular monoamine transporter using (+)-α-[11C] dihydrotetrabenazine (DTBZ) and positron emission tomography. J Cereb Blood Flow Metab. 1997;17:919–31.
Zhou Y, Sojkova J, Resnick SM, Wong DF. Relative equilibrium plot improves graphical analysis and allows bias correction of SUVR in quantitative [11C] PiB PET studies. J Nucl Med. 2012;53:622.
Farde L, Eriksson L, Blomquist G, Halldin C. Kinetic analysis of central [11C] raclopride binding to D2-dopamine receptors studied by PET—a comparison to the equilibrium analysis. J Cereb Blood Flow Metab. 1989;9:696–708.
Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–27.
Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–8.
La Joie R, Bejanin A, Fagan AM, Ayakta N, Baker SL, Bourakova V, et al. Associations between [(18)F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology. 2018;90:e282–e90. https://doi.org/10.1212/wnl.0000000000004860.
Firouzian A, Whittington A, Searle GE, Koychev I, Zamboni G, Lovestone S, et al. Imaging Aβ and tau in early stage Alzheimer’s disease with [18 F] AV45 and [18 F] AV1451. EJNMMI Res. 2018;8:19.
Jonasson M, Wall A, Chiotis K, Saint-Aubert L, Wilking H, Sprycha M, et al. Tracer kinetic analysis of (S)-18F-THK5117 as a PET tracer for assessing tau pathology. J Nucl Med. 2016;57:574–81.
Guehl NJ, Wooten DW, Yokell DL, Moon S-H, Dhaynaut M, Katz S, et al. Evaluation of pharmacokinetic modeling strategies for in-vivo quantification of tau with the radiotracer [18 F] MK6240 in human subjects. Eur J Nucl Med Mol Imaging. 2019;46:2099–111.
Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–47.
Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–72.
Chen K, Roontiva A, Thiyyagura P, Lee W, Liu X, Ayutyanont N, et al. Improved power for characterizing longitudinal amyloid-β PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. J Nucl Med. 2015;56:560–6.
Southekal S, Devous MD, Kennedy I, Navitsky M, Lu M, Joshi AD, et al. Flortaucipir F 18 quantitation using parametric estimation of reference signal intensity. J Nucl Med. 2018;59:944–51.
Brendel M, Högenauer M, Delker A. Alzheimer’s Disease Neuroimaging Initiative improved longitudinal [(18) F]-AV45 amyloid PET by white matter reference and VOI-based partial volume effect correction. Neuroimage. 2015;108:450–9.
Fleisher AS, Joshi AD, Sundell KL, Chen Y-F, Kollack-Walker S, Lu M, et al. Use of white matter reference regions for detection of change in florbetapir positron emission tomography from completed phase 3 solanezumab trials. Alzheimers Dement. 2017;13:1117–24.
Bilgel M, Beason-Held L, An Y, Zhou Y, Wong DF, Resnick SM. Longitudinal evaluation of surrogates of regional cerebral blood flow computed from dynamic amyloid PET imaging. J Cereb Blood Flow Metab. 2019. https://doi.org/10.1177/0271678X19830537.
Sojkova J, Beason-Held L, Zhou Y, An Y, Kraut MA, Ye W, et al. Longitudinal cerebral blood flow and amyloid deposition: an emerging pattern? J Nucl Med. 2008;49:1465–71.
Joseph-Mathurin N, Su Y, Blazey TM, Jasielec M, Vlassenko A, Friedrichsen K, et al. Utility of perfusion PET measures to assess neuronal injury in Alzheimer’s disease. Alzheimer's Dement: Diagn, Assess Dis Monitor. 2018;10:669–77.
Peretti DE, García DV, Reesink FE, Doorduin J, de Jong BM, De Deyn PP, et al. Diagnostic performance of regional cerebral blood flow images derived from dynamic PIB scans in Alzheimer’s disease. EJNMMI Res. 2019;9:1–9.
McCluskey SP, Plisson C, Rabiner EA, Howes O. Advances in CNS PET: the state-of-the-art for new imaging targets for pathophysiology and drug development. Eur J Nucl Med Mol Imaging. 2020; 47:451–89. https://doi.org/10.1007/s00259-019-04488-0.
Davis RE, Vanover KE, Zhou Y, Brašić JR, Guevara M, Bisuna B, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT 2A and dopamine D 2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology. 2015;232:2863–72.
Vanover KE, Davis RE, Zhou Y, Ye W, Brašić JR, Gapasin L, et al. Dopamine D 2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44:598–605.
Zhou Y, Ye W, Brašić JR, Crabb AH, Hilton J, Wong DF. A consistent and efficient graphical analysis method to improve the quantification of reversible tracer binding in radioligand receptor dynamic PET studies. Neuroimage. 2009;44:661–70.
Zhou Y, Ye W, Brašić JR, Wong DF. Multi-graphical analysis of dynamic PET. Neuroimage. 2010;49:2947–57.
Zhou Y, Endres CJ, Brašić JR, Huang S-C, Wong DF. Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. Neuroimage. 2003;18:975–89.
Shcherbinin S, Schwarz AJ, Joshi A, Navitsky M, Flitter M, Shankle WR, et al. Kinetics of the tau PET tracer 18F-AV-1451 (T807) in subjects with normal cognitive function, mild cognitive impairment, and Alzheimer disease. J Nucl Med. 2016;57:1535–42.
Brendel M, Wagner L, Levin J, Zach C, Lindner S, Bartenstein P, et al. Perfusion-phase [18F] THK5351 tau-PET imaging as a surrogate marker for neurodegeneration. J Alzheimer's Dis Rep. 2017;1:109–13.
Beyer L, Nitschmann A, Barthel H, van Eimeren T, Unterrainer M, Sauerbeck J, et al. Early-phase [18 F] PI-2620 tau-PET imaging as a surrogate marker of neuronal injury. Eur J Nucl Med Mol Imaging. 2020:1–12.
Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440–52.
Endres CJ, Hammoud DA, Pomper MG. Reference tissue modeling with parameter coupling: application to a study of SERT binding in HIV. Phys Med Biol. 2011;56:2499.
Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. Positron emission tomography quantification of [11C]-DASB binding to the human serotonin transporter: modeling strategies. J Cereb Blood Flow Metab. 2001;21:1342–53.
Zhou Y, Huang S-C, Bergsneider M. Linear ridge regression with spatial constraint for generation of parametric images in dynamic positron emission tomography studies. IEEE Trans Nucl Sci. 2001;48:125–30.
Logan J, Fowler JS, Volkow ND, Wang G-J, Ding Y-S, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40.
Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. https://doi.org/10.1212/WNL.43.11.2412-a.
Bidaut LM, Vallée JP. Automated registration of dynamic MR images for the quantification of myocardial perfusion. J Magn Reson Imaging. 2001;13:648–55.
Yan S, Zheng C, Paranjpe MD, Li J, Benzinger TLS, Lu J, et al. Association of sex and APOE ε4 with brain tau deposition and atrophy in older adults with Alzheimer's disease. Theranostics. 2020;10(23):10563–72.
Liu M, Paranjpe MD, Zhou X, Duy PQ, Goyal MS, Benzinger TLS, et al. Sex modulates the ApoE epsilon4 effect on brain tau deposition measured by (18)F-AV-1451 PET in individuals with mild cognitive impairment. Theranostics. 2019;9:4959–70. https://doi.org/10.7150/thno.35366.
Paranjpe MD, Chen X, Liu M, Paranjpe I, Leal JP, Wang R, et al. The effect of ApoE epsilon4 on longitudinal brain region-specific glucose metabolism in patients with mild cognitive impairment: a FDG-PET study. Neuroimage Clin. 2019;22:101795. https://doi.org/10.1016/j.nicl.2019.101795.
Cohen J. Statistical Power Analysis for the Behavioral Sciences–Second Edition. Hillsdale: 12 Lawrence Erlbaum Associates Inc; 1988. p. 13.
Chand GB, Dwyer DB, Erus G, Sotiras A, Varol E, Srinivasan D, et al. Two distinct neuroanatomical subtypes of schizophrenia revealed using machine learning. Brain. 2020;143:1027–38.
Sullivan GM, Feinn R. Using effect size—or why the P value is not enough. J Grad Med Educ. 2012;4:279–82.
Giavarina D. Understanding bland altman analysis. Biochem Med. 2015;25:141–51.
Bunce C. Correlation, agreement, and Bland–Altman analysis: statistical analysis of method comparison studies. Am J Ophthalmol. 2009;148:4–6.
Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–10.
Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. J R Statist Soc: Series D (Statist). 1983;32:307–17.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. https://doi.org/10.1016/0022-3956(75)90026-6.
Visser D, Wolters EE, Verfaillie SC, Coomans EM, Timmers T, Tuncel H, et al. Tau pathology and relative cerebral blood flow are independently associated with cognition in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2020;47:3165–75.
Rodriguez-Vieitez E, Leuzy A, Chiotis K, Saint-Aubert L, Wall A, Nordberg A. Comparability of [18F] THK5317 and [11C] PIB blood flow proxy images with [18F] FDG positron emission tomography in Alzheimer’s disease. J Cereb Blood Flow Metab. 2017;37:740–9.
Leuzy A, Rodriguez-Vieitez E, Saint-Aubert L, Chiotis K, Almkvist O, Savitcheva I, et al. Longitudinal uncoupling of cerebral perfusion, glucose metabolism, and tau deposition in Alzheimer's disease. Alzheimers Dement. 2018;14:652–63.
Veronese M, Bodini B, García-Lorenzo D, Battaglini M, Bongarzone S, Comtat C, et al. Quantification of [11C] PIB PET for imaging myelin in the human brain: a test—retest reproducibility study in high-resolution research tomography. J Cereb Blood Flow Metab. 2015;35:1771–82.
**ong YY, Mok V. Age-related white matter changes. J Aging Res. 2011;2011.
Lopresti B, Klunk W, Bi W, Cohen A, Mathis C, Price J. Use of pons as a normalizing region for [C-11] PIB PET scans: effect on subject classification. Alzheimer's Dement. 2011;7:7.
Tolboom N, Yaqub M, Boellaard R, Luurtsema G, Windhorst AD, Scheltens P, et al. Test-retest variability of quantitative [11 C] PIB studies in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36:1629–38.
Zhou Y, Sojkova J, Resnick SM, Wong DF. Relative equilibrium plot improves graphical analysis and allows bias correction of standardized uptake value ratio in quantitative 11C-PiB PET studies. J Nucl Med. 2012;53:622–8. https://doi.org/10.2967/jnumed.111.095927.
Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2016;160:134–47.
Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Absolute 10-year risk of dementia by age, sex and APOE genotype: a population-based cohort study. Cmaj. 2018;190:E1033–E41.
Bickeböller H, Campion D, Brice A, Amouyel P, Hannequin D, Didierjean O, et al. Apolipoprotein E and Alzheimer disease: genotype-specific risks by age and sex. Am J Hum Genet. 1997;60:439.
Acknowledgments
This study was partially supported by NIH P41EB025815, 1RO1HL150891-01, UF1AG032438, P50 AG05681, 2P01AG003991-36, P01AG026276, U19 AG032438-08, U01AG042791, and Mallinckrodt Institute of Radiology, Washington University in St. Louis School of Medicine.
Author information
Authors and Affiliations
Contributions
The study was designed by Yun Zhou. Material preparation, data collection, and analysis were performed by Yun Zhou, Shaney Flores, and Tammie L. S. Benzinger. The first draft of this manuscript was written by Yun Zhou. All authors read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology – Dementia
Rights and permissions
About this article
Cite this article
Zhou, Y., Flores, S., Mansor, S. et al. Spatially constrained kinetic modeling with dual reference tissues improves 18F-flortaucipir PET in studies of Alzheimer disease. Eur J Nucl Med Mol Imaging 48, 3172–3186 (2021). https://doi.org/10.1007/s00259-020-05134-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-05134-w