Abstract
Purpose
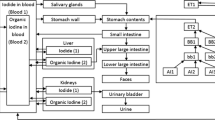
A considerable amount of radioiodine is exhaled after radioiodine therapy leading to unwanted radiation exposure through inhalation. This study focused on the concentration of radioactivity exhaled and its chemical nature.
Methods
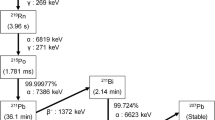
Air exhaled by 47 patients receiving 131I-iodine for different thyroid diseases (toxic goitre n = 26, Graves’ disease n = 13, thyroid cancer n = 8) was investigated with a portable constant air-flow sampler. Different chemical iodine species were collected separately (organic, elemental and aerosolic) up to 26 h after administration of the radioiodine capsule. The data approximated to a monoexponential time–activity curve when integrated over 100 h. The radioactivity in the filters was measured with a well counter at defined time points after administration.
Results
The radioactivity of 131I in the exhaled air 1 h after administration ranged from 1 to 100 kBq/m3. Two parameters (half-life of radioiodine exhalation and time-integrated activity over 100 h) were substantially higher in patients with cancer after near-total thyroidectomy (11.8 ± 2.1 h and 535 ± 140 kBq / m3, respectively) than in patients with hyperfunctioning thyroid tissue due to toxic adenoma (7.6 ± 2.5 h and 115 ± 27 kBq / m3, respectively) or Graves’ disease (6.4 ± 3.6 h and 113 ± 38 kBq / m3, respectively). The percentage of radioiodine in the exhaled air in relation to radioiodine administered to the patient was between 80 ppm and 150 ppm. The fraction of organically bound radioiodine (mean value) for all time points after administration was 94–99.9%. This percentage did not depend on the type of thyroid disease.
Conclusion
The amount of exhaled radioiodine is small but by no means negligible on the first day after administration. This is the first study to provide experimental evidence on a systematic basis that radioiodine becomes exhalable in vivo, i.e. in the patient. The mechanism of organification of orally administered radioiodine remains to be investigated.




Similar content being viewed by others
References
Salvatori M, Luster M. Radioiodine therapy dosimetry in benign thyroid disease and differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2010;37:821–8.
Verburg FA, Dietlein M, Lassmann M, Luster M, Reiners C. Why radioiodine remnant ablation is right for most patients with differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2009;36:343–6.
Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJ, et al.; European Association of Nuclear Medicine (EANM). Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:1941–59.
Stahl AR, Freudenberg L, Bockisch A, Jentzen W. A novel view on dosimetry-related radionuclide therapy: presentation of a calculatory model and its implementation for radioiodine therapy of metastasized differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2009;36:1147–55.
Kobe C, Weber I, Eschner W, Sudbrock F, Schmidt M, Dietlein M, Schicha H. Graves’ disease and radioiodine therapy. Is success of ablation dependent on the choice of thyreostatic medication? Nucl Med 2008;47:153–6.
Kobe C, Eschner W, Wild M, Rahlff I, Sudbrock F, Schmidt M, et al. Radioiodine therapy of benign thyroid disorders: what are the effective thyroidal half-life and uptake of 131I? Nucl Med Commun. 2010;31:201–5.
Mulazimoglu M, Edis N, Tamam MO, Uyanik E, Ozpacaci T. The evaluation of the external dose measurement of the patients treated with radioiodine therapy. Radiat Prot Dosimetry. 2010;141:233–8.
Flux GD, Haq M, Chittenden SJ, Buckley S, Hindorf C, Newbold K, et al. A dose-effect correlation for radioiodine ablation in differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2010;37:270–5.
Barrington SF, Kettle AG, O’Doherty MJ, Wells CP, Somer EJ, Coakley AJ. Radiation dose rates from patients receiving iodine-131 therapy for carcinoma of the thyroid. Eur J Nucl Med. 1996;23:123–30. Erratum in: Eur J Nucl Med 1997;24:1545.
Barrington SF, O’Doherty MJ, Kettle AG, Mountford PJ. Dose rate measurements combined with social behavior models enables estimation of potential radiation hazards to families of patients treated with radioiodine. Health Phys. 2000;78:100–1.
Barrington SF, Anderson P, Kettle AG, Gadd R, Thomson WH, Batchelor PJ. Measurement of the internal dose to families of outpatients treated with 131I for hyperthyroidism. Eur J Nucl Med Mol Imaging. 2008;35:2097–104.
Barrington SF, O’Doherty MJ, Kettle AG, Thomson WH, Mountford PJ, Burrell DN, et al. Radiation exposure of the families of outpatients treated with radioiodine (iodine-131) for hyperthyroidism. Eur J Nucl Med. 1999;26:686–92.
Pant GS, Sharma SK, Bal CS, Kumar R, Rath GK. Radiation dose to family members of hyperthyroidism and thyroid cancer patients treated with 131I. Radiat Prot Dosimetry. 2006;118:22–7.
Lassmann M, Hänscheid H, Schelper LF, Körber C, Reiners C. Measurement of incorporation in family members of radioiodine therapy patients after therapy of benign thyroid diseases. Nucl Med. 1998;37:120–3.
Nishizawa K, Ohara K, Ohshima M, Maekoshi H, Orito T, Watanabe T. Monitoring of I excretions and used materials of patients treated with 131I. Health Phys. 1980;38:467–81.
Reiners C, Lassmann M. Radioiodine (131I) treatment of hyperthyroidism: radiation protection and quality assurance. Eur J Nucl Med. 1999;26:683–5.
Krześniak JW, Chomicki OA, Czerminska M, Gorowski T. Airborne radioiodine contamination caused by I-131 treatment. Nucl Med. 1979;18:246–51.
Krześniak JW, Fill H, Oberladstätter M. Radioactivity levels of the air inhaled by personnel of a radioiodine therapy unit. Nucl Med. 1987;26:143–6.
Heinemann K, Vogt KJ, Friedrich W, Feinendegen LE. Monitoring of radioiodine in nuclear medical therapy. Strahlentherapie. 1983;159:490–4.
Schomäcker K, Fischer T, Eschner W, Gaidouk MI, Schicha H. Exhalation of I-131 after radioiodine therapy (RIT): time dependence and chemical form. Nucl Med. 2001;40:15–22.
Deuber H, Wilhelm JG. Occurrence of penetrating iodine species in the exhaust air of PWR power plants. In: First MW, editor. Proceedings of the 16th DOE Nuclear Air Cleaning Conference. CONF-801038, vol 2; 1981. p. 1354–86.
Wilhelm JG, Deuber H, Wilhelm JG, Deuber H. The removal of short-lived iodine isotopes. In: Goossens W, editor. Treatment of gaseous effluents at nuclear facilities. Chur: Harwood; 1991. p. 67–96.
Wilhelm JG. Development and application of filters for air cleaning in nuclear power plants. Nucl Eng Des. 1987;103:139–47.
Wellner U, Eschner W, Hillger HW, Schicha H. Exposure of relatives of patients after stationary radioiodine therapy by inhalation of 131I in their homes. Nucl Med. 1998;37:113–9.
Sudbrock F, Schomäcker K, Fischer T, Zimmermanns B, Dietlein M, Kobe C, et al. Exhalation of I-131 after radioiodine therapy: measurements in exhaled air and dosimetric considerations. Nucl Med. 2011;2:A71.
Sudbrock F, Boldt F, Kobe C, Hammes J, Eschner W, Schicha H. Radiation exposure of patients after application of radiopharmaceuticals. Part 2: therapeutic procedures. Nucl Med. 2009;48(1):17–25.
Gründel M, Kopka B, Schulz R. 131I exhalation by patients undergoing therapy of thyroid diseases. Radiat Prot Dosimetry. 2008;129:435–8.
Acknowledgment
The authors gratefully acknowledge the support of Miss H. Quraischy in collecting the patient data.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Klaus Schomäcker and Ferdinand Sudbrock contributed equally to this work.
Rights and permissions
About this article
Cite this article
Schomäcker, K., Sudbrock, F., Fischer, T. et al. Exhalation of 131I after radioiodine therapy: measurements in exhaled air. Eur J Nucl Med Mol Imaging 38, 2165–2172 (2011). https://doi.org/10.1007/s00259-011-1888-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1888-8