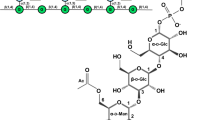
Abstract
The efficiency of de novo synthesis of hyaluronic acid (HA) using Pasteurella multocida hyaluronate synthase (PmHAS) is limited by its low catalytic activity during the initial reaction steps when monosaccharides are the acceptor substrates. In this study, we identified and characterized a β-1,4-N-acetylglucosaminyl-transferase (EcGnT) derived from the O-antigen gene synthesis cluster of Escherichia coli O8:K48:H9. Recombinant β1,4 EcGnT effectively catalyzed the production of HA disaccharides when the glucuronic acid monosaccharide derivative 4-nitrophenyl-β-D-glucuronide (GlcA-pNP) was used as the acceptor. Compared with PmHAS, β1,4 EcGnT exhibited superior N-acetylglucosamine transfer activity (~ 12-fold) with GlcA-pNP as the acceptor, making it a better option for the initial step of de novo HA oligosaccharide synthesis. We then developed a biocatalytic approach for size-controlled HA oligosaccharide synthesis using the disaccharide produced by β1,4 EcGnT as a starting material, followed by stepwise PmHAS-catalyzed synthesis of longer oligosaccharides. Using this approach, we produced a series of HA chains of up to 10 sugar monomers. Overall, our study identifies a novel bacterial β1,4 N-acetylglucosaminyltransferase and establishes a more efficient process for HA oligosaccharide synthesis that enables size-controlled production of HA oligosaccharides.
Key points
• A novel β-1,4-N-acetylglucosaminyl-transferase (EcGnT) from E. coli O8:K48:H9.
• EcGnT is superior to PmHAS for enabling de novo HA oligosaccharide synthesis.
• Size-controlled HA oligosaccharide synthesis relay using EcGnT and PmHAS.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Chen Y, Thon V, Li Y, Yu H, Ding L, Lau K, Qu J, Hie L, Chen X (2011) One-pot three-enzyme synthesis of UDP-GlcNAc derivatives. Chem Commun (Camb) 47(38):10815–10817. https://doi.org/10.1039/c1cc14034e
Chen QX, Shao XT, Ling PX, Liu F, Han GY, Wang FS (2017a) Recent advances in polysaccharides for osteoarthritis therapy. Eur J Med Chem 139:926–935. https://doi.org/10.1016/j.ejmech.2017.08.048
Chen XF, Chen R, Yu XX, Tang DY, Yao WB, Gao XD (2017b) Metabolic engineering of Bacillus subtilis for biosynthesis of heparosan using heparosan synthase from Pasteurella multocida, PmHS1. Bioproc Biosyst Eng 40(5):675–681. https://doi.org/10.1007/s00449-016-1732-4
Cozikova D, Silova T, Moravcova V, Smejkalova D, Pepeliaev S, Velebny V, Hermannova M (2017) Preparation and extensive characterization of hyaluronan with narrow molecular weight distribution. Carbohyd Polym 160:134–142. https://doi.org/10.1016/j.carbpol.2016.12.045
DeAngelis PL (1996) Enzymological characterization of the Pasteurella multocida hyaluronic acid synthase. Biochemistry 35(30):9768–9771. https://doi.org/10.1021/bi960154k
DeAngelis PL (1999) Molecular directionality of polysaccharide polymerization by the Pasteurella multocida hyaluronan synthase. J Biol Chem 274(37):26557–26562. https://doi.org/10.1074/jbc.274.37.26557
Dosio F, Arpicco S, Stella B, Fattal E (2016) Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv Drug Deliver Rev 97:204–236. https://doi.org/10.1016/j.addr.2015.11.011
Feldman MF, Marolda CL, Monteiro MA, Perry MB, Parodi AJ, Valvano MA (1999) The activity of a putative polyisoprenol-linked sugar translocase (Wzx) involved in Escherichia coli O antigen assembly is independent of the chemical structure of the O repeat. J Biol Chem 274(49):35129–35138. https://doi.org/10.1074/jbc.274.49.35129
Fu X, Shang WJ, Wang SS, Liu YP, Qu JY, Chen X, Wang PG, Fang JQ (2017) A general strategy for the synthesis of homogeneous hyaluronan conjugates and their biological applications. Chem Commun 53(25):3555–3558. https://doi.org/10.1039/c6cc09431g
Green DE, DeAngelis PL (2017) Identification of a chondroitin synthase from an unexpected source, the green sulfur bacterium Chlorobium phaeobacteroides. Glycobiology 27(5):469–476. https://doi.org/10.1093/glycob/cwx008
Guan W, Cai L, Wang PG (2010) Highly efficient synthesis of UDP-GalNAc/GlcNAc analogues with promiscuous recombinant human UDP-GalNAc pyrophosphorylase AGX1. Chemistry 16(45):13343–13345. https://doi.org/10.1002/chem.201002315
Iguchi A, Iyoda S, Kikuchi T, Ogura Y, Katsura K, Ohnishi M, Hayashi T, Thomson NR (2015) A complete view of the genetic diversity of the Escherichia coli O-antigen biosynthesis gene cluster. DNA Res 22(1):101–107. https://doi.org/10.1093/dnares/dsu043
**g W, DeAngelis PL (2004) Synchronized chemoenzymatic synthesis of monodisperse hyaluronan polymers. J Biol Chem 279(40):42345–42349. https://doi.org/10.1074/jbc.M402744200
Kang Z, Zhou ZX, Wang Y, Huang H, Du GC, Chen J (2018) Bio-based strategies for producing glycosaminoglycans and their oligosaccharides. Trends Biotechnol 36(8):806–818. https://doi.org/10.1016/j.tibtech.2018.03.010
Li Y, Yu H, Thon V, Chen Y, Muthana MM, Qu J, Hie L, Chen X (2014) Donor substrate promiscuity of the N-acetylglucosaminyltransferase activities of Pasteurella multocida heparosan synthase 2 (PmHS2) and Escherichia coli K5 KfiA. Appl Microbiol Biotechnol 98(3):1127–1134. https://doi.org/10.1007/s00253-013-4947-1
Li J, Su GW, Liu J (2017) Enzymatic synthesis of homogeneous chondroitin sulfate oligosaccharides. Angew Chem Int Edit 56(39):11784–11787. https://doi.org/10.1002/anie.201705638
Li JE, Su GW, Sparkenbaugh E, Pawlinski R, Liu J (2018) Enzymatic synthesis of homogeneous chondroitin sulfate e oligosaccharides. Glycobiology 28(12):1083–1083
Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T (2016) Hyaluronic acid in inflammation and tissue regeneration. Wounds 28(3):78–88
Liu J, Linhardt RJ (2014) Chemoenzymatic synthesis of heparan sulfate and heparin. Nat Prod Rep 31(12):1676–1685. https://doi.org/10.1039/c4np00076e
Liu L, Liu Y, Li J, Du G, Chen J (2011) Microbial production of hyaluronic acid: current state, challenges, and perspectives. Microb Cell Fact 10:99. https://doi.org/10.1186/1475-2859-10-99
Liu KF, Guo L, Chen XL, Liu LM, Gao C (2023) Microbial synthesis of glycosaminoglycans and their oligosaccharides. Trends Microbiol 31(4):369–383. https://doi.org/10.1016/j.tim.2022.11.003
Masuko S, Bera S, Green DE, Weiwer M, Liu J, DeAngelis PL, Linhardt RJ (2012) Chemoenzymatic synthesis of uridine diphosphate-GlcNAc and uridine diphosphate-GalNAc analogs for the preparation of unnatural glycosaminoglycans. J Org Chem 77(3):1449–1456. https://doi.org/10.1021/jo202322k
Meng DH, Du RR, Chen LZ, Li MT, Liu F, Hou J, Shi YK, Wang FS, Sheng JZ (2019) Cascade synthesis of uridine-5’-diphosphate glucuronic acid by coupling multiple whole cells expressing hyperthermophilic enzymes. Microb Cell Fact 18(1):118. https://doi.org/10.1186/s12934-019-1168-z
Otto NJ, Green DE, Masuko S, Mayer A, Tanner ME, Linhardt RJ, DeAngelis PL (2012) Structure/Function analysis of Pasteurella multocida heparosan synthases toward defining enzyme specificity and engineering novel catalysts. J Biol Chem 287(10):7203–7212. https://doi.org/10.1074/jbc.M111.311704
Papakonstantinou E, Roth M, Karakiulakis G (2012) Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol 4(3):253–258. https://doi.org/10.4161/derm.21923
Qiu Y, Ma Y, Huang Y, Li S, Xu H, Su E (2021) Current advances in the biosynthesis of hyaluronic acid with variable molecular weights. Carbohydr Polym 269:118320. https://doi.org/10.1016/j.carbpol.2021.118320
Rojas-Macias MA, Stahle J, Lutteke T, Widmalm G (2015) Development of the ECODAB into a relational database for Escherichia coli O-antigens and other bacterial polysaccharides. Glycobiology 25(3):341–347. https://doi.org/10.1093/glycob/cwu116
Senchenkova SN, Guo X, Naumenko OI, Shashkov AS, Perepelov AV, Liu B, Knirel YA (2016) Structure and genetics of the O-antigens of Escherichia coli O182–O187. Carbohydr Res 435:58–67. https://doi.org/10.1016/j.carres.2016.09.014
Shashkov AS, Wang T, Perepelov AV, Weintraub A, Liu B, Widmalm G, Knirel YA (2015) Structure elucidation and biosynthesis gene cluster organization of the O-antigen of Escherichia coli O170. Carbohydr Res 417:11–14. https://doi.org/10.1016/j.carres.2015.08.013
Sheng J, Liu R, Xu Y, Liu J (2011) The dominating role of N-deacetylase/N-sulfotransferase 1 in forming domain structures in heparan sulfate. J Biol Chem 286(22):19768–19776. https://doi.org/10.1074/jbc.M111.224311
Stenutz R, Weintraub A, Widmalm G (2006) The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol Rev 30(3):382–403. https://doi.org/10.1111/j.1574-6976.2006.00016.x
Vaidyanathan D, Williams A, Dordick JS, Koffas MAG, Linhardt RJ (2017) Engineered heparins as new anticoagulant drugs. Bioeng Transl Med 2(1):17–30. https://doi.org/10.1002/btm2.10042
Veith AP, Henderson K, Spencer A, Univer ADOBE, Baker AB (2019) Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliver Rev 146:97–125. https://doi.org/10.1016/j.addr.2018.09.010
Wang TT, Zhu CY, Zheng S, Meng CC, Wang TT, Meng DH, Li YJ, Zhu HM, Wang FS, Sheng JZ (2018) Identification and characterization of a chondroitin synthase from Avibacterium paragallinarum. Appl Microbiol Biot 102(11):4785–4797. https://doi.org/10.1007/s00253-018-8926-4
Wang TT, Deng JQ, Chen LZ, Sun L, Wang FS, Ling PX, Sheng JZ (2020) The second member of the bacterial UDP-N-acetyl-D-glucosamine:heparosan alpha-1, 4-N-acetyl-D-glucosaminyltransferase superfamily: GaKfiA from Gallibacterium anatis. Int J Biol Macromol 147:170–176. https://doi.org/10.1016/j.ijbiomac.2020.01.016
Whittaker DV, Parolis LA, Parolis H (1994) Escherichia coli K48 capsular polysaccharide: a glycan containing a novel diacetamido sugar. Carbohydr Res 256(2):289–301. https://doi.org/10.1016/0008-6215(94)84214-0
Williams KJ, Halkes KM, Kamerling JP, DeAngelis PL (2006) Critical elements of oligosaccharide acceptor substrates for the Pasteurella multocida hyaluronan synthase. J Biol Chem 281(9):5391–5397. https://doi.org/10.1074/jbc.M510439200
Xu YM, Cai C, Chandarajoti K, Hsieh PH, Li LY, Pham TQ, Sparkenbaugh EM, Sheng JZ, Key NS, Pawlinski R, Harris EN, Linhardt RJ, Liu J (2014) Homogeneous low-molecular-weight heparins with reversible anticoagulant activity. Nat Chem Biol 10(4):248-+ https://doi.org/10.1038/Nchembio.1459
Yao ZY, Qin J, Gong JS, Ye YH, Qian JY, Li H, Xu ZH, Shi JS (2021) Versatile strategies for bioproduction of hyaluronic acid driven by synthetic biology. Carbohydr Polym 264:118015. https://doi.org/10.1016/j.carbpol.2021.118015
Yin FX, Wang FS, Sheng JZ (2016) Uncovering the catalytic direction of chondroitin AC exolyase: from the reducing end towards the non-reducing end. J Biol Chem 291(9):4399–4406. https://doi.org/10.1074/jbc.C115.708396
Yuan P, Lv M, ** P, Wang M, Du G, Chen J, Kang Z (2015) Enzymatic production of specifically distributed hyaluronan oligosaccharides. Carbohydr Polym 129:194–200. https://doi.org/10.1016/j.carbpol.2015.04.068
Zhang X, Lin L, Huang H, Linhardt RJ (2020) Chemoenzymatic synthesis of glycosaminoglycans. Accounts Chem Res 53(2):335–346. https://doi.org/10.1021/acs.accounts.9b00420
Zhang YS, Gong JS, Yao ZY, Jiang JY, Su C, Li H, Kang CL, Liu L, Xu ZH, Shi JS (2022) Insights into the source, mechanism and biotechnological applications of hyaluronidases. Biotechnol Adv 60:108018. https://doi.org/10.1016/j.biotechadv.2022.108018
Acknowledgements
This work was supported in part by Projects of Science and Technology Department of Shandong Province (Project no. 2020CXGC010602), the National Key R&D Program of China (Project no. 2021YFC2103102), National Natural Science Foundation of China (Project no. 31770845), Open Projects Fund of NMPA Key Laboratory for Quality Research and Evaluation of Carbohydrate-based Medicine, and Shandong Key Laboratory of Carbohydrate Chemistry and Glycobiology.
Author information
Authors and Affiliations
Contributions
JS designed and coordinated the work. Jiu-Ying Sun carried out the experiments. Jiu-Ying Sun and JD carried out the HPLC analysis. RD and ZL carried out the donor experiments. Jiu-Ying Sun, SX, and YC carried out the synthesis and purification of oligosaccharides. XG and FW conducted MS and NMR analyses. JS and Jiu-Ying Sun wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, JY., Deng, JQ., Du, RR. et al. Novel β1,4 N-acetylglucosaminyltransferase in de novo enzymatic synthesis of hyaluronic acid oligosaccharides. Appl Microbiol Biotechnol 107, 5119–5129 (2023). https://doi.org/10.1007/s00253-023-12671-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12671-5