Abstract
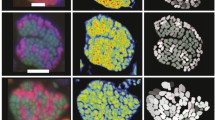
A mixed culture from an anaerobic biowaste digester was enriched on propionate and used to investigate interspecies hydrogen transfer in dependence of spatial distances between propionate degraders and methanogens. From 20.3 mM propionate, 20.8 mM acetate and 15.5 mM methane were formed. Maximum specific propionate oxidation and methane formation rates were 49 and 23 mmol mg−1 day−1, respectively. Propionate oxidation was inhibited by only 20 mM acetate by about 50 %. Intermediate formate formation during inhibited methanogensis was observed. The spatial distribution and the biovolume fraction of propionate degraders and of methanogens in relation to the total population during aggregate formation were determined. Measurements of interbacterial distances were conducted with fluorescence in situ hybridization by application of group-specific 16S rRNA-targeted probes and 3D image analyses. With increasing incubation time, floc formation and growth up to 54 μm were observed. Propionate degraders and methanogens were distributed randomly in the flocs. The methanogenic biovolume fraction was high at the beginning and remained constant over 42 days, whereas the fraction of propionate degraders increased with time during propionate feeding. Interbacterial distances between propionate degraders and methanogens decreased with time from 5.30 to 0.29 μm, causing an increase of the maximum possible hydrogen flux from 1.1 to 10.3 nmol ml−1 min−1. The maximum possible hydrogen flux was always higher than the hydrogen formation and consumption rate, indicating that reducing the interspecies distance by aggregation is advantageous in complex ecosystems.







Similar content being viewed by others
References
Amani T, Nosrati M, Mousavi SM (2012) Response surface methodology analysis of anaerobic syntrophic degradation of volatile fatty acids in an upflow anaerobic sludge bed reactor inoculated with enriched cultures. Biotechnol Bioproc Eng 17:133–144
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial population. Appl Environ Microbiol 56:1919–1925
Batstone DJ, Picioreanu C, van Loosdrecht MCM (2006) Multidimensional modeling to investigate interspecies hydrogen transfer in anaerobic biofilms. Wat Res 40:3099–3108
Bleicher K, Winter J (1994) Formate production and utilization by methanogens and by sewage sludge consortia—interference with the concept of interspecies formate transfer. Appl Microbiol Biotechnol 40:910–915
Boone DR, Bryant MP (1980) Propionate-degrading bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl Environ Microbiol 40:626–632
Bryant MP, Wolin EA, Wolin MJ, Wolfe RS (1967) “Methanobacillus omelanskii”, a symbiotic association of two species of bacteria. Arch Microbiol 59:20–31
Chen S, Liu X, Dong X (2005) Syntrophobacter sulfatireducens sp. nov., a novel syntrophic, propionate-oxidizing bacterium isolated from UASB reactors. Int J Syst Evol Microbiol 55:1319–1324
Conrad R, Schink B, Phelps TJ (1986) Thermodynamics of H2-consuming and H2-producing metabolic reactions in diverse methanogenic environments under in situ conditions. FEMS Microbiol Ecol 38:353–360
Daims H, Lücker S, Wagner M (2006) daime, a novel image analysis program for microbial ecology and biofilm research. Environ Microbiol 8:200–213
De Bok FAM, Stams AJM, Dijkema C, Boone DR (2001) Pathway of propionate oxidation by syntrophic culture of Smithella propionica and Methanospirillum hungatei. Appl Environ Microbiol 67:1800–1804
De Bok FAM, Plugge CM, Stams AJM (2004) Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res 38:1368–1375
de Bok FAM, Harmsen HJM, Plugge CM, de Vries MC, Akkermans DL, de Vos WM, Stams AJM (2005) The first true obligately syntrophic propionate-oxidizing bacterium, Pelotomaculum schinkii sp. nov., co-cultured with Methanospirillum hungatei, and emended description of the genus Pelotomaculum. Int J Syst Evol Microbiol 55:1697–1703
Dong X, Stams AJM (1995) Evidence for H2 and formate formation during syntrophic butyrate and propionate degradation. Anaerobe 1:35–39
Dwyer DF, Weeg-Aerssens E, Shelton DR, Tiedje JM (1988) Bioenergetic conditions of butyrate metabolism by a syntrophic, anaerobic bacterium in coculture with hydrogen-oxidizing methanogenic and sulfidogenic bacteria. Appl Environ Microbiol 54:1354–4359
Felchner-Zwirello M, Winter J, Gallert C (2012) Mass spectrometric identification of 13C- labeled metabolites during anaerobic propionic acid oxidation. Chem Biodiv 9:376–384
Gallert C, Winter J (2005) Bacterial metabolism in wastewater treatment systems. In: Jördening HJ, Winter J (eds) Environmental biotechnology—concept and applications. Wiley, Weinheim, pp 1–48
Gallert C, Winter J (2008) Propionic acid accumulation and degradation during restart of a full-scale anaerobic biowaste digester. Bioresour Technol 99:170–178
Gallert C, Henning A, Winter J (2003) Scale-up of anaerobic digestion of the biowaste fraction from domestic wastes. Wat Res 37:1433–1441
Gerardi MH (2003) The microbiology of anaerobic digesters. Wiley, New Jersey
Gujer W, Zehnder AJB (1983) Conversion processes in anaerobic digestion. Wat Sci Technol 15:127–167
Harmsen HJM, Kengen HMP, Akkermans ADL, Stams AJM (1995) Phylogenetic analysis of two syntrophic propionate-oxidizing bacteria in enrichment cultures. Syst Appl Microbiol 18:67–73
Harmsen HJM, Akkermans ADL, Stams AJM, De Vos WM (1996) Population dynamics of propionate-oxidizing bacteria under methanogenic and sulfidogenic conditions in anaerobic granular sludge. Appl Environ Microbiol 62:2163–2168
Harmsen HJM, Van Kuijk BLM, Plugge CM, Akkermans ADL, De Vos WM, Stams AJM (1998) Syntrophobacter fumaroxidans sp. nov., a syntrophic propionate-degrading sulfate-reducing bacterium. Int J Syst Bacteriol 48:1383–1387
Imachi H, Sekiguchi Y, Kamagata Y, Ohashi A, Harada H (2000) Cultivation and in situ detection of a thermophilic bacterium capable of oxidizimg propionate in syntrophic association with hydrogenotrophic methanogens in a thermophilic methanogenic granular sludge. Appl Environ Microbiol 66:3608–3615
Imachi H, Sekiguchi Y, Kagamata Y, Hanada S, Ohashi A, Harada H (2002) Pelotomaculum thermopropionicum gen. nov., sp. nov., an anaerobic, thermophilic, syntrophic propionate- oxidizing bacterium. Int J Syst Evol Microbiol 52:1729–1735
Inanc B, Matsui S, Ide S (1999) Propionic acid accumulation in anaerobic digestion of carbohydrates: an investigation on the role of hydrogen gas. Wat Sci Tech 40:93–100
Ishii S, Kosaka T, Hori K, Hotta Y, Watanabe K (2005) Coaggregation facilitates interspecies hydrogen transfer between Pelotomaculum thermopropionicum and Methanothermobacter thermoatotrophicus. Appl Environ Microbiol 71:7838–7845
Lens PNL, O'Flaherty V, Dijkema C, Colleran E, Stams AJM (1996) Propionate degradation by mesophilic anaerobic sludge: degradation pathways and effects of other volatile fatty acids. J Ferment Bioeng 82:387–391
Liu Y, Balkwill DL, Aldrich HC, Drake GR, Boone RD (1999) Characterization of the anaerobic propionate degrading syntrophs Smithella propionica gen. nov., sp. nov. and Syntrophobacter wolinii. Int J Syst Bacteriol 49:545–556
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mawson AJ, Earle RL, Larsen VF (1991) Degradation of acetic and propionic acids in the methane fermentation. Water Res 12:1549–1554
McCarty PL, Smith DP (1986) Anaerobic wastewater treatment. Environ Sci Technol 20:1200–1206
Müller N, Worm P, Schink B, Stams AJM, Plugge CM (2010) Syntrophic butyrate and propionate oxidation processes: from genomes to reaction mechanisms. Environ Microbiol Rep 2:489–499
Nielsen H, Daims H, Lemmer H (2009) FISH handbook for biological wastewater treatment: Identification and quantification of microorganisms in activated sludge and biofilms by FISH. IWA, London
Nilsen RK, Torsvik T, Lien T (1996) Desulfotomaculum thermocisternum sp. nov., a sulfate reducer isolated from a hot North Sea oil reservoir. Int J Syst Bacteriol 46:397–402
Plugge CM, Dijkema C, Stams AJM (1993) Acetyl–CoA cleavage pathway in a syntrophic propionate oxidizing bacterium growing on fumarate in the absence of methanogens. Microbiol Lett 110:71–76
Plugge CM, Balk M, Stams AJM (2002) Desulfotomaculum thermobenzoicum subsp. thermosyntrophicum subsp. nov., a thermophilic, syntrophic, propionate-oxidizing, spore-forming bacterium. Int J Syst Evol Microbiol 52:391–399
Samain E, Dubourguier HC, Albagnac G (1984) Isolation and characterization of Desulfobulbus elongatus sp. nov. from a mesophilic industrial digester. Syst Appl Microbiol 5:391–401
Schink B (1992) Syntrophism among prokaryotes. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer K-H (eds) The prokaryotes, 3rd edn. Springer, New York, pp 276–299
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev 61:262–280
Schmidt JE, Ahring BK (1993) Effects of hydrogen and formate on the degradation of propionate and butyrate in thermophilic granules from an upflow anaerobic sludge blanket reactor. Appl Environ Microbiol 59:2546–2551
Schmidt JE, Ahring BK (1995) Interspecies electron transfer during propionate and butyrate degradation in mesophilic, granular sludge. Appl Environ Microbiol 61:2765–2767
Scholten JCM, Conrad R (2000) Energetics of syntrophic propionate oxidation in defined batch and chemostat cocultures. Appl Environ Microbiol 66:2934–2942
Shin SG, Lee S, Lee C, Hwang K, Hwang S (2010) Qualitative and quantitative assessment of microbial community in batch anaerobic digestion of secondary sludge. Biores Technol 101:9461–9470
Shi-yi L, Jian C (1992) The contribution of interspecies hydrogen transfer to the substrate removal in methanogenesis. Process Biochem 27:285–289
Sieber JR, McInerney MJ, Gunsalus RP (2012) Genomic insights into syntrophy: the paradigm for anaerobic metabolic cooperation. Annu Rev Microbiol 66:429–452
Stahl DA, Amann R (1991) Development and application of nucleic acid probes. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 205–248
Stams AJM (1994) Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Van Leeuwenhoek 66:271–294
Stams AJM, Dong X (1995) Role of formate and hydrogen in the degradation of propionate and butyrate by defined suspended cocultures of acetogenic and methanogenic bacteria. Antonie Van Leeuwenhoek 68:281–284
Stams AJM, Kremer DR, Nicolay K, Weenk G, Hansen AT (1984) Pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol 139:167–173
Stams AJM, Grothenius JTC, Zehnder AJB (1989) Structure–function relationship in granular sludge. In: Hattori T, Ishida Y, Maruyama R, Morita R, Uchida A (eds) Recent advances in microbial ecology. Japan Scientific Societies, Tokyo, pp 440–445
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Thiele JH, Zeikus JG (1988) Control of interspecies electron flow during anaerobic digestion: significance of formate transfer during syntrophic methanogenesis in flocs. Appl Environ Microbiol 54:20–29
Thiele JH, Chartrain M, Zeikus JG (1988) Control of interspecies electron flow during anaerobic digestion: role of floc formation in syntrophic methanogenesis. Appl Environ Microbiol 54:10–19
Van Kuijk BLM, Stams AJM (1995) Sulfate reduction by a syntrophic propionate-oxidizing bacterium. In: Proceedings of the International Meeting on Anaerobic Processes for Bioenergy and Environment, part 5, Copenhagen, 25–27 January 1995
Wallrabenstein C, Hauschild E, Schink B (1994) Pure culture and cytological properties of Syntrophobacter wolini. FEMS Microbiol Lett 123:249–254
Wallrabenstein C, Hauschild E, Schink B (1995) Syntrophobacter pfennigii sp. nov., new syntrophically propionate-oxidizing anaerobe growing in pure culture with propionate and sulfate. Arch Microbiol 164:346–352
Wang L, Zhou Q, Li FT (2006) Avoiding propionic acid accumulation in the anaerobic process for biohydrogen production. Biomass Bioenergy 30:177–182
Widdel F, Pfennig N (1982) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. II. Incomplete oxidation of propionate by Desulfobulbus propionicus gen. nov., sp. nov. Arch Microbiol 131:360–365
Wolin MJ (1975) Interactions between H2-producing and methane-producing species. In: Schlegel HG, Gottschalk G, Pfennig N (eds) Symposium on microbial production and utilization of gases. Goltz, Göttingen, pp 141–150
Wu WM, Jain MK, de Macario EC, Thiele JH, Zeikus JG (1992) Microbial composition and characterization of prevalent methanogens and acetogens isolated from syntrophic methanogenic granules. Appl Microbiol Biotechnol 38:282–290
Acknowledgments
This work has been conducted within the project on “Propionic acid metabolism during biowaste treatment using different digester regimes” (GA 546/4-2) sponsored by Deutsche Forschungsgemeinschaft, Bonn, Germany. Financial support came also from Karlsruhe Institute of Technology (KIT) within the STUB excellence initiative project “Anaerobic propionic acid degradation in syntrophic consortia- microbial community distribution”, conducted together with Water Chemistry at Engler Bunte Institut of KIT. Special thanks go to Heiko Schwegmann for help with the ApoTom.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Felchner-Zwirello, M., Winter, J. & Gallert, C. Interspecies distances between propionic acid degraders and methanogens in syntrophic consortia for optimal hydrogen transfer. Appl Microbiol Biotechnol 97, 9193–9205 (2013). https://doi.org/10.1007/s00253-012-4616-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4616-9