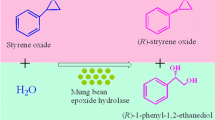
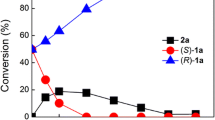
Abstract
Enantiopure epoxides are high value-added synthons for the production of pharmaceuticals, agrochemicals, as well as versatile fine chemicals and have broad scope of market demand for their applications. A major challenge in conventional organic synthesis is to generate such compounds in high enantiopurity with reasonable yield. Among possible chemical and biological technologies for enantiopure epoxide preparation, enzymatic kinetic resolution has been paid much attention with respect to its high enantioselectivity. Epoxide hydrolase (EH) has shown promising characteristics for the preparation of enantiopure epoxides and vicinal diols during enantioselective hydrolysis of racemic epoxides. EH is readily available from microbial resources thus it is being employed for biohydrolysis of a variety of epoxides. Recent technical progress in EH-catalyzed enantioselective hydrolysis is summarized in terms of exploration of novel EH, its functional improvement, high throughput assay, and preparative scale resolution process.



Similar content being viewed by others
References
Archelas A, Furstoss R (1999) Biocatalytic approaches for the synthesis of enantiopure epoxides. Top Curr Chem 200:159–191
Archelas A, Furstoss R (2001) Synthetic applications of epoxide hydrolases. Curr Opin Chem Biol 5:112–119
Badalassi F, Wahler D, Klein G, Crotti P, Reymond JL (2000) A versatile periodate-coupled fluorogenic assay for hydrolytic enzymes. Angew Chem Int Ed 39:4067–4070
Baldascini H, Ganzeveld KJ, Janssen DB, Beenackers AACM (2001) Effect of mass transfer limitations on the enzymatic kinetic resolution of epoxides in a two-liquid-phase system. Biotechnol Bioeng 73:44–53
Besse P, Veschambre H (1994) Chemical and biological synthesis of enantiopure epoxides. Tetrahedron 50:8885–8927
Bottalla A, Ibrahim-Ouali M, Santelli M, Furstoss R, Archelas A (2007) Epoxide hydrolase-catalysed kinetic resolution of a spiroepoxide, a key building block of various 11-heterosteroids. Adv Synth Catal 349:1102–1110
Cao L, Lee J, Chen JW, Wood TK (2006) Enantioconvergent production of (R)-1-phenyl-1, 2-ethanediol from styrene oxide by combining the Solanum tuberosum and an evolved Agrobacterium radiobacter AD1 epoxide hydrolases. Biotechnol Bioeng 94:522–529
Cedrone F, Niel S, Ait-abdelkader N, Torre C, Krumm H, Roca S, Bhatnagar T, Maichele A, Reetz MT, Baratti JC (2003) Directed evolution of the epoxide hydrolase from Aspergillus niger. Biocatal Biotrans 21:357–364
Cedrone F, Bhatnagar T, Baratti JC (2005) Colorimetric assays for quantitative analysis and screening of epoxide hydrolase activity. Biotechnol Lett 27:1921–1927
Choi WJ, Choi CY (2005) Production of chiral epoxides: epoxide hydrolase-catalyzed enantioselective hydrolysis. Biotechnol Bioprocess Eng 10:167–179
Choi WJ, Lee EY, Yoon SJ, Choi CY (1999a) Biocatalytic production of chiral epichlorohydrin in organic solvent. J Biosci Bioeng 88:339–341
Choi WJ, Choi CY, de Bont JAM, Weijers CAGM (1999b) Resolution of 1, 2-epoxyhexane by Rhodotorula glutinis using a two-phase membrane bioreactor. Appl Microbiol Biotechnol 53:7–11
Choi WJ, Choi CY, de Bont JAM, Weijers CAGM (2000) Continuous production of enantiopure 1, 2-epoxyhexane by yeast epoxide hydrolase in a two-phase membrane bioreactor. Appl Microbiol Biotechnol 54:641–646
Choi WJ, Puah SM, Tan LL, Ng SS (2008) Production of (R)-ethyl-3, 4-epoxybutyrate by newly isolated Acinetobacter baumannii containing epoxide hydrolase. Appl Microbiol Biotechnol 79:61–67
Deregnaucourt J, Archelas A, Barbirato F, Paris J, Furstoss R (2006) Enzymatic transformations; 61. Preparation of enantiopure trifluoromethyl-substituted aromatic epoxides and vicinal diols using the Aspergillus niger epoxide hydrolase-catalysed resolution. Adv Synth Catal 348:1165–1169
Devi AV, Lahari C, Swarnalatha L, Fadnavis NW (2008) Gelozymes in organic synthesis. part IV: resolution of glycidate esters with crude mung bean (Phaseolus radiatus) epoxide hydrolase immobilized in gelatin matrix. Tetrahedron: Asymmetry 19:1139–1144
de Vries EJ, Janssen DB (2003) Biocatalytic conversion of epoxides. Curr Opin Biotechnol 14:414–420
Doumèche B, Archelas A, Furstoss R (2006) Enzymatic transformations 62. Preparative scale synthesis of enantiopure glycidyl acetals using an Aspergillus niger epoxide hydrolase-catalysed kinetic resolution. Adv Synth Catal 348:1948–1957
DuBois GC, Appella E, Levin W, Lu AYH, Jerina DM (1978) Hepatic microsomal epoxide hydrase. Involvement of a histidine at the active site suggests a nucleophilic mechanism. J Biol Chem 253:2932–2939
Elenkov MM, Tang L, Meetsma A, Hauer B, Janssen DB (2008) Formation of enantiopure 5 substituted oxazolidinones through enzyme-catalysed kinetic resolution of epoxides. Org Lett 10:2417–2420
Faber K, Kroutil W (2002) Stereoselectivity in biocatalytic enantioconvergent reactions and a computer program for its determination. Tetrahedron: Asymmetry 13:377–382
Fluxá VS, Wahler D, Reymond J (2008) Enzyme assay and activity fingerprinting of hydrolases with the red-chromogenic adrenaline test. Nature Protocols 3:1270–1277
Fu**o A, Sugai T (2008) Chemoenzymatic approach to enantiomerically pure (R)-3-hydroxy-3-methyl-4-pentenoic acid ester and its application to a formal total synthesis of taurospongin A. Adv Synth Catal 350:1712–1716
Fu**o A, Asano M, Yamaguchi H, Shirasaka N, Sakoda A, Ikunaka M (2007) Bacillus subtilis epoxide hydrolase-catalyzed preparation of enantiopure 2-methylpropane-1, 2, 3-triol monobenzyl ether and its application to expeditious synthesis of (R)-bicalutamide. Tetrahedron Lett 48:979–983
Furuhashi K (1992) Chirality in industry, pp. 167–186. Wiley, New York
Hammock BD, Pinot F, Beetham JK, Grant DF, Arand ME, Oesch F (1994) Isolation of a putative hydroxyacyl enzyme intermediate of an epoxide hydrolase. Biochem Biophys Res Comm 198:850–856
Hasnaoui-Dijoux G, Elenkov MM, Lutje Spelberg JH, Hauer B, Janssen DB (2008) Catalytic promiscuity of halohydrin dehalogenase and its application in enantioselective epoxide ring opening. ChemBioChem 9:1048–1051
Hwang S, Hyun H, Lee B, Park Y, Choi C, Han J, Joo H (2006) Screening from the genome databases: novel epoxide hydrolase from Caulobacter crescentus. J Microbiol Biotechnol 16:32–36
Janssen DB, Majerić-Elenkov M, Hasnaoui G, Hauer B, Spelberg JHL (2006) Enantioselective formation and ring-opening of epoxides catalysed by halohydrin dehalogenases. Biochem Soc Trans 34:291–295
Jia X, Wang Z, Li Z (2008) Preparation of (S)-2-, 3-, and 4-chlorostyrene oxides with the epoxide hydrolase from Sphingomonas sp. HXN-200. Tetrahedron: Asymmetry 19:407–415
Johnson RA, Sharpless KB (1993) Catalytic asymmetric epoxidation of allylic alcohols. In: Ojima I (ed) Catalytic asymmetric synthesis. VCH, New York, pp 103–158
Karboune S, Archelas A, Furstoss R, Baratti J (2005) Immobilization of epoxide hydrolase from Aspergillus niger onto DEAE-cellulose: enzymatic properties and application for the enantioselective resolution of a racemic epoxide. J Mol Catal B: Enzym 32:175–183
Kim HS, Lee SJ, Lee EJ, Hwang JW, Park S, Kim SJ, Lee EY (2005) Cloning and characterization of a fish microsomal epoxide hydrolase of Danio rerio and application to kinetic resolution of racemic styrene oxide. J Mol Catal B: Enzym 37:30–35
Kim HS, Lee OK, Lee SJ, Hwang S, Kim SJ, Yang S, Park S, Lee EY (2006) Enantioselective epoxide hydrolase activity of a newly isolated microorganism, Sphingomonas echinoides EH-983, from seawater. J Mol Catal B: Enzym 41:130–135
Kotik M, Kyslík P (2006) Purification and characterisation of a novel enantioselective epoxide hydrolase from Aspergillus niger M200. Biochim Biophys Acta 1760:245–252
Kotik M, Brichac J, Kyslík P (2005) Novel microbial epoxide hydrolases for biohydrolysis of glycidyl derivatives. J Biotechnol 120:364–375
Kroutil W, Mischitz M, Plachota P, Faber K (1996) Deracemization of (±)-cis-2, 3-epoxyheptane via enantioconvergent biocatalytic hydrolysis using Nocardia EH1-epoxide hydrolase. Tetrahedron Lett 46:8379–8382
Labuschagne M, Albertyn J (2007) Cloning of an epoxide hydrolase-encoding gene from Rhodotorula mucilaginosa and functional expression in Yarrowia lipolytica. Yeast 24:69–78
Lacourciere GM, Armstrong RN (1993) The catalytic mechanism of microsomal epoxide hydrolase involves an ester intermediate. J Am Chem Soc 115:10466–10467
Lee EY (2008) Epoxide hydrolase-mediated enantioconvergent bioconversions to prepare chiral epoxides and alcohols. Biotechnol Lett 30:1509–1514
Lee EY, Shuler ML (2007) Molecular engineering of epoxide hydrolase and its application to asymmetric and enantioconvergent hydrolysis. Biotechnol Bioeng 98:318–327
Lee SJ, Kim HS, Kim SJ, Park S, Kim BJ, Shuler ML (2007) Cloning, expression and enantioselective hydrolytic catalysis of a microsomal epoxide hydrolase from a marine fish, Mugil cephalus. Biotechnol Lett 29:237–246
Liu Y, Sha Q, Wu S, Wang J, Yang L, Sun W (2006a) Enzymatic resolution of racemic phenyloxirane by a novel epoxide hydrolase from Aspergillus niger SQ-6 and its fed-batch fermentation. J Ind Microbiol Biotechnol 33:274–282
Liu Z, Michel J, Wang Z, Witholt B, Li Z (2006b) Enantioselective hydrolysis of styrene oxide with the epoxide hydrolase of Sphingomonas sp. HXN-200. Tetrahedron: Asymmetry 17:47–52
Liu Z, Li Y, Xu Y, ** L, Zheng Y (2007) Cloning, sequencing, and expression of a novel epoxide hydrolase gene from Rhodococcus opacus in Escherichia coli and characterization of enzyme. Appl Microbiol Biotechnol 74:99–106
Mateo C, Archelas AA, Fernandez-Lafuente R, Guisan JM, Furstoss R (2003) Enzymatic transformations. Immobilized Aspergillus niger epoxide hydrolase as a novel biocatalytic tool for repeated-batch hydrolytic kinetic resolution of epoxides. Org Biomol Chem 1:2739–2743
Monfort N, Archelas A, Furstoss R (2004) Enzymatic transformations. Part 55: highly productive epoxide hydrolase catalysed resolution of an azole antifungal key synthon. Tetrahedron 60:601–605
Monterde ML, Lombard M, Archelas A, Cronin A, Arand M, Furstoss R (2004) Enzymatic transformation. Part 58: enantioconvergent biohydrolysis of styrene oxide derivatives catalyzed by the Solanum tuberosum epoxide hydrolase. Tetrahedron: Asymmetry 15:2801–2805
Morisseau C, Nellaiah H, Archelas A, Furstoss R, Baratti JC (1997) Asymmetric hydrolysis of racemic para-nitrostyrene oxide using an epoxide hydrolase preparation from Aspergillus niger. Enzyme Microb Technol 20:446–452
Moussou P, Archelas A, Baratti J, Furstoss R (1998) Microbiological transformations. 38. Clues to the involvement of a general acid activation during hydrolysis of para-substituted styrene oxides by a soluble epoxide hydrolase from Syncephalastrum racemosum. J Org Chem 63:3532–3537
Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, Sussman JL, Verschueren KHG, Goldman A (1992) The α/β hydrolase fold. Protein Eng 5:197–211
Pedragosa-Moreau S, Morisseau C, Zylber J, Archelas A, Baratti J, Furstoss R (1996) Microbiological transformations. 33. Fungal epoxide hydrolases applied to the synthesis of enantiopure para-substituted styrene oxides. A mechanistic approach. J Org Chem 61:7402–7407
Petri A, Marconcini P, Salvadori P (2005) Efficient immobilization of epoxide hydrolase onto silica gel and use in the enantioselective hydrolysis of racemic para-nitrostyrene oxide. J Mol Catal B: Enzym 32:219–224
Pienaar DP, Mitra RK, Deventer TI, Botes AL (2008) Synthesis of a variety of optically active hydroxylated heterocyclic compounds using epoxide hydrolase technology. Tetrahedron Lett 49:6752–6755
Reetz MT (2006) Directed evolution of enantioselective enzymes as catalysts for organic synthesis. Adv Catal 49:1–69
Reetz MT, Wang L (2006) High-throughput selection system for assessing the activity of epoxide hydrolases. Comb Chem High Throughput Screen 9:295–299
Reetz MT, Torre C, Eipper A, Lohmer R, Hermes M, Brunner B, Maichele A, Bocola M, Arand M, Cronin A, Genzel Y, Archelas A, Furstoss R (2004) Enhancing the enantioselectivity of an epoxide hydrolase by directed evolution. Org Lett 6:177–180
Reetz MT, Wang L, Bocola M (2006) Directed evolution of enantioselective enzymes: iterative cycles of CASTing for probing protein-sequence space. Angew Chem Int Ed 45:1236–1241
Rink R, Janssen DB (1998) Kinetic mechanism of the enantioselective conversion of styrene oxide by epoxide hydrolase from Agrobacterium radiobacter AD1. Biochemistry 37:18119–18127
Rink R, Spelberg JHL, Pieters RJ, Kingma J, Nardini M, Kellogg RM, Dijkstra BW, Janssen DB (1999) Mutation of tyrosine residues involved in the alkylation half reaction of epoxide hydrolase from Agrobacterium radiobacter AD1 results in improved enantioselectivity. J Am Chem Soc 121:7417–7418
Rui L, Cao L, Chen W, Reardon KF, Wood TK (2005) Protein engineering of epoxide hydrolase from Agrobacterium radiobacter AD1 for enhanced activity and enantioselective production of (R)-1-phenylethane-1, 2-diol. Appl Environ Microbiol 71:3995–4003
Schrader W, Eipper A, Pugh DJ, Reetz MT (2002) Second-generation MS based high-throughput screening system for enantioselective catalysis and biocatalysis. Can J Chem 80:626–632
Simeó Y, Faber K (2006) Selectivity enhancement of enantio- and stereo-complementary epoxide hydrolases and chemo-enzymatic deracemization of (±)-2-methylglycidyl benzyl ether. Tetrahedron: Asymmetry 17:402–409
Smith JG (1984) Synthetically useful reactions of epoxides. Synthesis 629–656
Steinreiber A, Faber K (2001) Microbial epoxide hydrolases for preparative biotransformations. Curr Opin Biotechnol 12:552–558
Takagi M, Uemura N, Furuhashi K (1990) Microbial transformation processes of aliphatic-hydrocarbons. Ann N Y Acad Sci 613:697–701
Tokunaga M, Larrow JF, Kakiuchi F, Jacobsen EN (1997) Asymmetric catalysis with water: efficient kinetic resolution of terminal epoxides by means of catalytic hydrolysis. Science 277:936–938
van der Werf MJ, de Bont JAM, Swarts HJ (1999) Acid-catalyzed enzymatic hydrolysis of 1-methylcyclohexene oxide. Tetrahedron: Asymmetry 10:4225–4230
van Loo B, Spelberg JHL, Kingma J, Sonke T, Wubbolts MG, Janssen DB (2004) Directed evolution of epoxide hydrolase from A. radiobacter toward higher enantioselectivity by error-prone PCR and DNA shuffling. Chem Biol 11:981–990
van Loo B, Kingma J, Arand M, Wubbolts MG, Janssen DB (2006) Diversity and biocatalytic potential of epoxide hydrolases identified by genome analysis. Appl Environ Microbiol 72:2905–2917
van Loo B, Kingma J, Heyman G, Wittenaar A, Spelberg JHL, Sonke T, Janssen DB (2009) Improved enantioselective conversion of styrene epoxides and meso-epoxides through epoxide hydrolases with a mutated nucleophile-flanking residue. Enzyme Microb Technol 44:145–153
Wahler D, Reymond JL (2002) The adrenaline test for enzymes. Angew Chem Int Ed 41:1229–1232
Weijers CAGM, de Bont JAM (1999) Epoxide hydrolases from yeasts and other sources: versatile tools in biocatalysis. J Mol Catal B: Enzym 6:199–214
Weijers CAGM, Meeuwse P, Herpers RLJM, Franssen MCR, Sudhölter EJR (2005) Stereoselectivity and substrate specificity in the kinetic resolution of methyl-substituted 1-oxaspiro[2.5]octanes by Rhodotorula glutinis epoxide hydrolase. J Org Chem 70:6639–6646
Wu S, Shen J, Zhou X, Chen J (2007) A novel enantioselective epoxide hydrolase for (R)-phenyl glycidyl ether to generate (R)-3-phenoxy-1, 2-propanediol. Appl Microbiol Biotechnol 76:1281–1287
Xu W, Xu JH, Pan J, Gu O, Wu XY (2006) Enantioconvergent hydrolysis of styrene epoxides by newly discovered epoxide hydrolases in mung bean. Org Lett 8:1737–1740
Yoo SS, Park S, Lee EY (2008) Enantioselective resolution of racemic styrene oxide at high concentration using recombinant Pichia pastoris expressing epoxide hydrolase of Rhodotorula glutinis in the presence of surfactant and glycerol. Biotechnol Lett 30:1807–1810
Zocher F, Enzelberger MM, Bornscheuer UT, Hauer B, Schmid RD (1999) A colorimetric assay suitable for screening epoxide hydrolase activity. Analytica Chimica Acta 391:345–351
Acknowledgments
This work was supported by Science and Engineering Research Council of A*STAR (Agency for Science, Technology and Research), Singapore.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, W.J. Biotechnological production of enantiopure epoxides by enzymatic kinetic resolution. Appl Microbiol Biotechnol 84, 239–247 (2009). https://doi.org/10.1007/s00253-009-2110-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2110-9